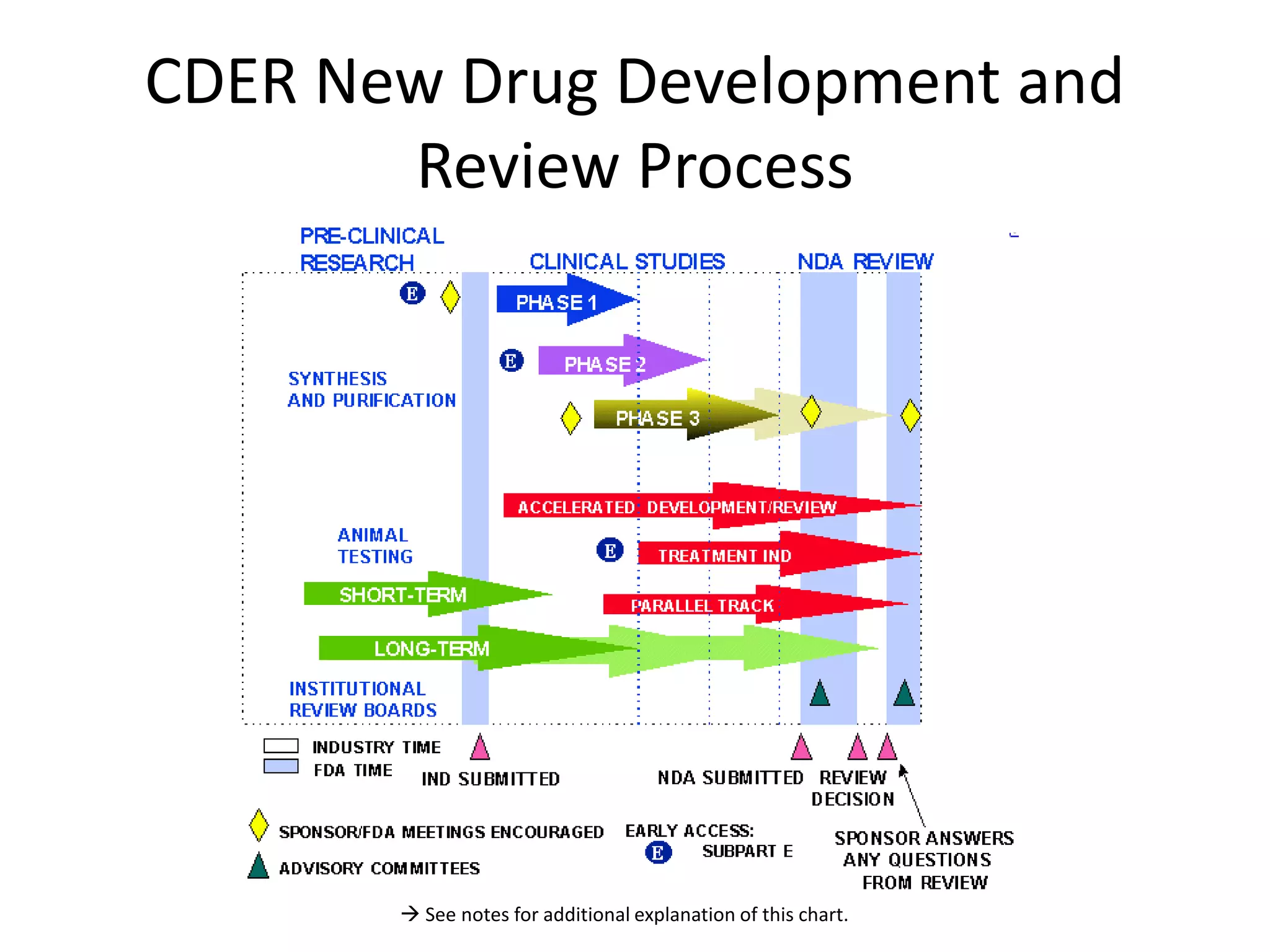
The document outlines the drug development process, emphasizing the significant costs and time involved, which averages around 12 years from drug synthesis to FDA approval at a cost of approximately $1.3 billion. It highlights the role of IT in supporting business processes to optimize efficiency in bringing new treatments to market, and discusses the impact of generics on product revenue post-patent expiration. Additionally, various strategies and metrics related to drug development and clinical trial phases are presented to illustrate the industry's financial dynamics.