Transposition of Great Arteries
- 1. TRANSPOSITON OF GREAT ARTERIES Dr. Harshil Joshi UNMICRC
- 2. INTRODUCTION TGA - most common etiology for cyanotic congenital heart disease in the newborn. 5-7% of all patients with congenital heart disease. Overall annual incidence is 20-30 per 100,000 live births, TGA is isolated in 90% of patients Rarely associated with syndromes or extracardiac malformations.
- 3. • “There is probably more controversy surrounding the definition of ‘transposition’ than any other term in paediatric cardiology” (Anderson-Pathology of Congenital heart disease 1981)
- 4. Definition Malposition- “when the great arteries exhibit abnormal spatial relation but concordant ventricles of origin, they are said to be Malposed, not Transposed.”
- 5. Definition Farre coined the term “transposition” of the aorta and pulmonary artery in 1814 “the aorta is arising out of the right ventricle and pulmonary artery is coming from left ventricle.”
- 6. Definition •TGA-Origin of Aorta above the morphologically right ventricle and the pulmonary artery above the morphologically left ventricle. •DORV-Origin of both great arteries entirely or predominantly above the RV •DOLV- Origin of both great arteries entirely or predominantly above the LV •ACM-Origin of malposed Ao above the LV and origin of malposed PA above the RV.
- 7. NORMAL EMBRYOLOGIC DEVELOPMENT The superior end of primitive RV is initially continuous with conus cordis and truncus that will eventually give rise to both aortic and pulmonary outflow.
- 8. Complicated realignment causes the right side of canal to move and align with future RA & RV. During the same time the AV canal is divided into right and left canals by growth of superior and inferior endocardial cushions which meet to form the septum intermedium.
- 9. Venrticular Septation The two primitive ventricles begin to dilate with continuous growth of myocardium outside and traberculae formation on the inside with resultant muscular IVS between them. The growth of muscular IVS halts in middle of 7th week before its leading edge meets the septum intermedium. The space between the free rim of muscular IVS and fused endocardial cushions is called the IVF, later closed by membranous IVS. Septum intermedium Muscular IVS
- 10. Truncal & Conal septation During 5th week opposing ridges appear in cephalic part of truncus- right superior and left inferior truncal swellings (neural crest origin) They grow towards each other in a spiral manner foreshadowing the future course of Aorto-pulmonary septum. Simultaneously right dorsal and left ventral conal cushions develop.
- 11. The proximal extent of right conus cushion terminates at the superior border of right AV orifice. With conal cushion fusion the septum divides the conus into Anterolateral outflow tract of RV and posteromedial outflow tract of LV.
- 12. The IVF found above muscular IVS is reduced in size with completion of conus septum. Proliferation of inferior AV cushions along top rim of muscular IVS and fusion with abutting conus septum results in complete closure of IVF and membranous IVS results
- 13. EMBRYOGENIC THEORIES •Straight truncoconal septum hypothesis incriminating the abnormal septation of aorta and pulmonary trunk. •Abnormal fibrous skeleton hypothesis where PA- MV continuity occurs instead of AO-MV continuity. •Abnormal embryonic hemodynamic hypothesis caused by obstructive and altered flow characteristics. •Inverted truncal swelling theory citing inverted development below the semilunar valves.
- 15. DEFINITION • Complete transposition of the great arteries (TGA) is a congenital cardiac anomaly in which the aorta arises entirely or largely from the right ventricle (RV) and in which the pulmonary trunk arises entirely or largely from the left ventricle (LV), known as ventriculoarterial discordant connection.
- 18. Shunt
- 19. HISTORICAL NOTE The first morphologic description of TGA is attributed to Baillie in 1797. The term transposition of the aorta and pulmonary artery was coined by Farre. Surgery of TGA commenced in 1950 when Blalock and Hanlon at Johns Hopkins Hospital described a closed method of atrial septectomy. In 1953, Lillehei and Varco described a "partial physiologic correction" (or atrial switch) consisting of anastomosis of right pulmonary veins to right atrium and inferiorvena cava (IVC) to left atrium, a technique that became known as the "Baffes operation.
- 20. History cont…. • Palliation of TGA was revolutionized when Rashkind and Miller in Philadelphia introduced balloon atrial septostomy (BAS) in 1966. • Senning in 1959, who refashioned the walls of the right atrium and the atrial septum to accomplish atrial-level transposition of venous return. • The Mustard procedure, in which the atrial septum is excised and a pericardial baffle used to redirect systemic and pulmonary venous flow was successfully introduced at the Toronto Sick Children's Hospital in 1963 and reported in 1964.
- 21. • In 1969,Rastelli and colleagues combined intraventricular tunnel repair (LV to aorta) of the double outlet RV operation with a rerouting valved extracardiac conduit (RV to pulmonary artery) and closure of the origin of the pulmonary trunk from the LV to produce an anatomic repair of TGA, VSD, and LVOTO. • Idriss and colleagues attempted such a procedure in two patients with an intact ventricular septum in 1961 using cardiopulmonary bypass (CPB), transferring the great arteries and a ring of aorta carrying the coronary arteries. • Jatene and colleagues in Brazil achieved a major breakthrough in 1975 with the first successful use of an arterial switch procedure {Jatene procedure), applying it in infants with TGA and VSD. History cont….
- 22. MORPHOLOGY 1. Right Ventricle • The RV is normally positioned, hypertrophied, and large in TGA. • In about 90% of cases,the aorta is rightward and anterior and ascends parallel to the posterior and leftward pulmonary trunk. • There is less wedging of the pulmonary trunk between mitral and tricuspid valves in TGA than of the aorta in normal hearts. As a result, a larger area of contiguity exists between mitral and tricuspid valves than normally. • These atrioventricular (AV) valves may be at virtually the same level.
- 23. 2.Left Ventricle • Typically pulmonary-mitral fibrous continuity exists, comparable to aortic-mitral continuity in the normal heart. • In the normal heart the LV wall is same thickness as the RV wall in utero. After birth, LV wall thickness increases progressively, whereas the RV wall becomes relatively thinner. • In TGA the RV wall is considerably increases in thickness with age. When the ventricular septum is intact and no important pulmonary stenosis is present, the LV wall is of normal thickness at birth. Wall thickness remains static, however, leading to less than normal thickness within a few weeks of birth and a relatively thin wall by age 2 to 4 months.
- 24. • When a VSD is present, LV wall thickness increases slightly less than in the normal heart but remains well within the normal range during the first year of life.With LVOTO (pulmonary stenosis) the evolution is similar. • In infants with TGA the LV cavity is the usual ellipsoid in shape at birth but soon becomes banana shaped. Alteration in LV function accompanies this geometric change. • RV function is usually normal in TGA in the perinatal period. Thereafter, when the ventricular septum is intact, RV end-diastolic volume is increased and RV ejection fraction decreased. • Depressed RV ejection fraction is unlikely to be caused by increased afterload or decreased preload and probably results from depressed RV function from relative myocardial hypoxia or the geometry of the chamber.
- 25. 3. Atria • The atria are normally formed in TGA. Right atrial size is usually larger than normal, particularly when the ventricular septum is intact.
- 26. 4. Conduction System • The AV node and bundle of His lie in a normal position, although the AV node is abnormally shaped and may be partly engulfed in the right trigone. • The left bundle branch originates more distally from the bundle of His than usual.
- 27. RELATIONSHIP OF THE GREAT VESSELS •COMMONEST arrangement is aortic root is anterior and to the right of the pulmonary trunk. •However all possible permutations and relations may be viewed. •In almost all arrangements the aortic sinuses bearing the coronaries face the pulmonary artery.
- 28. Arterial relationships •Relationship of the great arteries and their valves • Aortic position around pulmonary valve (Bargeron,1981)
- 29. Coronary Anatomy Yacoub classification (Thorax 33;418, 1978) Type A: LCA from Lt. sinus & RCA from the Rt. sinus. Type B: Single coronary artery. Type C: Two para-commissural ostia with or without intramural course. Type D: RCA and circumflex take origin from the right ostium, LAD alone takes origin from the left ostium. Type E: RCA and LAD take origin from the left ostium, circumflex alone takes origin from the right ostium
- 30. Coronary Anatomy •Other than origin , important to note origin in relation to the sinuses- radial, tangential, vertical origin. •PROBLEMS •Eccentric origin •High origin •Tangential takeoff across the aortic wall •Crossing a commissure of the aortic valve •Intramural coronaries
- 32. Coexisting Cardiac Anomalies 1. Ventricular Septal Defect • The approximate distribution of VSD location includes perimembranous (33%), AV canal (5%), muscular (27%), malalignment (30%). • When septum is displaced to the right, the pulmonary trunk may be biventricular in origin and over a juxtapulmonary VSD and may be associated with subaortic stenosis or aortic arch obstruction (arch hypoplasia, coarctation, or interruption). • Posterior (leftward) malalignment is associated with varying degrees of LVOTO–subpulmonary stenosis, annular hypoplasia, or even pulmonary valvar atresia. • The conal septum may be absent or almost gone, and the VSD is then juxta-arterial (doubly committed).
- 33. 2. Left Ventricular Outflow Tract Obstruction • Development of LVOTO, which produces subpulmonary obstruction, is part of the natural history of many patients with TGA. • The obstruction may be dynamic or anatomic. • Dynamic type of LVOTO, developing in patients with TGA and intact ventricular septum, is the result of leftward bulging of the muscular ventricular septum secondary to higher RV than LV pressure. • The septum impinges against the anterior mitral leaflet in combination with abnormal systolic anterior leaflet motion (SAM). Thus, the mechanism is similar to that present in hypertrophic obstructive cardiomyopathy, but there is no asymmetric septal hypertrophy. • In patients with TGA and VSD, stenosis is usually subvalvar and valvar. Subvalvar stenosis is in the form of a localized fibrous ring, long tunnel- type flbromuscular narrowing, or muscular obstruction related to protrusion of the infundibular septum into the medial or anterior aspect of the LV outflow tract.
- 34. 3. Patent Ductus Arteriosus • Patent ductus arteriosus (PDA) is more common in hearts with TGA than in hearts with ventriculoarterial concordant connection. • Persistence of a large PDA for more than a few months is associated with an increased prevalence of peripheral vascular disease.
- 35. 4. Tricuspid Valve Anomalies • The tricuspid to mitral anulus circumference ratio, normally greater than 1, is less than 1 in 50% of patients. This reduced ratio is most marked in hearts with associated coarctation. • Functionally important tricuspid valve anomalies are present in only about 4% of surgical patients. • The tricuspid leaflets can be redundant and dysplastic in TGA. • Accessory tricuspid tissue may prolapse through the VSD and produce LVOTO. • The tricuspid anulus may be dilated, the valve may be hypoplastic in association with underdevelopment of the RV sinus. • Anular overriding or tensor straddling or both can occur.
- 36. 5. Mitral Valve Anomalies • Important structural anomalies of the mitral valve are present in 20% to 30% of hearts with TGA, mostly in combination with a VSD. • Mitral valve anomalies can be categorized into four groups, as those affecting the • Leaflets • Commissures • Chordae tendineae • Papillary muscles • The most important from a surgical standpoint are those of mitral valve overriding or straddling, in which the mitral valve leaflet is frequently also cleft.
- 37. 6. Aortic Obstruction • Coexisting aortic obstruction can be discrete (coarctation, or less often interrupted aortic arch) or caused by distal arch hypoplasia. • it occurs in 7% to 10% of patients with TGA and VSD. • This coexistence is more frequent when the VSD is juxtapulmonary and the pulmonary trunk is partly over the RV in association with rightward and anterior displacement of the infundibular septum and with some subaortic narrowing. • When there is associated coarctation, underdevelopment of the RV sinus is more common.
- 38. 7. Right Aortic Arch • Right aortic arch occurs in about 5% of patients with TGA. • It is more common when there is an associated VSD than when the ventricular septum is intact and when there is associated leftward juxtaposition of the atrial appendages.
- 39. 8. Leftward Juxtaposition of Atrial Appendages • Leftward juxtaposition of the atrial appendages occurs in about 2.5% of patients with TGA. • Bilateral conus and dextrocardia seem more common in TGA associated with leftward juxtaposition than in TGA generally.
- 40. 9. Right Ventricular Hypoplasia • RV hypoplasia was found to some degree in 17% of the necropsy series of TGA reported by Riemenschneider and colleagues.
- 41. 10. Abnormal pulmonary flow • The maldistribution is dependent on a common anatomic feature found in transposition, abnormal rightward inclination of the main pulmonary artery, which results in a straight ejection direction from left ventricle to main pulmonary artery to right pulmonary artery. • The abnormally increased distribution of pulmonary blood flow to the right lung in TGA often is associated with some degree of hypoplasia of the left pulmonary arterial vessels and is further manifested in the occasional reports of unilateral, always left-sided, pulmonary vein stenosis or hypoplasia
- 42. PATHOPHYSIOLOGY
- 43. PATHOPHYSIOLOGY Fetal circulation compatible with normal fetal survival and gestational development. • SVC Blood ---» TV ---» RV--- » ASC.AORTA • Blood reaching coronary and cerebral circulations have slightly lower blood glucose and Po2 than normal. • Blood reaching pulmonary circuit and descending aorta has better glucose conc. And higher Po2. But birth weight remains unaffected.
- 44. PATHOPHYSIOLOGY Birth ↓ PVR falls, SVR increases ↓ Two parallel circulation established. ↓ Desaturated blood gets more desturated, and oxegenated gets more oxygenated ↓ Problem begins…
- 45. PATHOPHYSIOLOGY Mixing must be equal and bidirectional Pumping Unsuitable pump for Systemic and Pulmonary bed
- 46. PATHOPHYSIOLOGY Mixing Concept of effective Pulmonary Blood Flow Concept of effective systemic blood flow The effective pulmonary blood flow, effective systemic blood flow, and net anatomic right-to-left and net anatomic left-to-right shunts are each equal to each other, and this volume is the intercirculatory mixing: the flow in TGA on which survival depends
- 48. PATHOPHYSIOLOGY Level of Mixing Intracardiac patent foramen ovale ASD VSD Extracardiac PDA bronchopulmonary collateral circulation
- 49. PATHOPHYSIOLOGY Mixing Direction of mixing Interatrial shunting Ventricular systole – Lt. to Rt. Ventricular Diastole- Rt. To Lt. Interventricular shunting Isovolumetric contraction- Lt. to Rt. Ventricular ejection- Rt. To Lt. Isovolumetric Relaxation- Rt. To Lt. Early diastole - Rt. To Lt. [Sommer RJ et al JACC 16; 1437,1990]
- 50. PATHOPHYSIOLOGY Pump Geometry End diastolic volume Systolic output Ejection Fraction Wall thickness
- 51. PATHOPHYSIOLOGY Pump following three conditions: Pure LV volume overload- TGA,IVS Pure LV pressure overload- TGA,IVS,PS LV Pressure +Volume overload- TGA,VSD with or without PS.
- 52. PATHOPHYSIOLOGY Pump Geometry LV- Spherical at birth Banana shaped later. RV- Crescent shape Spherical later.
- 53. PATHOPHYSIOLOGY
- 54. PATHOPHYSIOLOGY Pump End diastolic volume (Circulation 44;899, 1971) At birth it is normal for both LV and RV With IVS – RVEDV=normal in first month then increase LVEDV=normal initial 3-4months then ↑es=180% With VSD- RVEDV=increases from the first month 163+/-25% LVEDV=increases about 259% of normal With VSD,PS RVEDV=normal in first month then 124+/-26%% LVEDV=117% of normal RA volume ↑ to 181% but LA volume remains normal.
- 55. PATHOPHYSIOLOGY Pump Wall thickness( correlation with age) Normal Heart- RV- normal LV- Good TGA RV-potential (growth rate same in+/- VSD) LV- with increasing age growth rate is slow, being minimal for the hearts with IVS.
- 56. PULMONARY VASCULAR DISEASE The persistence of a large PDA in infants with intact ventricular septum has been implicated as a cause for increased pulmonary vascular disease as has prolonged hypoxemia or polycythemia increases in pulmonary vascular muscularity and intimal hyperplasia with vessel obstruction Intense systemic hypoxemia is commonly present, and local pulmonary hypoxemia can result from increased bronchial arterial vessels and bronchopulmonary anastomoses carrying hypoxemic systemic blood to the precapillary pulmonary arterioles. Thus, increased pulmonary vascular flow, pressure, and vasoconstrictive factors, possibly in association with abnormal platelet and red cell factors, can result in increased pulmonary vessel shear stress, endothelial damage, microthrombi, and the early induction and rapid progression of vascular disease.
- 57. Pathophysiology
- 58. Arterial Blood Gases and Metabolic Responses • pulmonary venous blood reflects chemoreceptor-stimulated hyperventilation • systemic arterial pO2 levels are rarely higher than 35 mm Hg, and the pCO2 is usually normal • Pulmonary pO2 levels may be increased to as high as 110 mm Hg and the pCO2 levels reduced to 15 to 25 mm Hg
- 59. Natural History and Presentation PHYSIOLOGICAL- CLINICAL CLASSIFICATION TGA (IVS or small VSD) with increased PBF and small ICS TGA (VSD large) with increased PBF and large ICS TGA (VSD and LVOTO),with restricted PBF TGA (VSD and PVOD), with restrictive PBF
- 60. TGA (IVS or Small VSD) Poor Mixing Includes infants without a VSD or with a VSD 3 mm or less in diameter A patent foramen ovale or naturally occurring atrial septal defect (ASD) is usually present. Cyanosis is apparent in half these infants within the first hour of life and in 90% within the first day and is rapidly progressive. The systemic arterial pO2 may be as low as 15 to 25 mm Hg at presentation, with resultant anaerobic glycolysis and severe metabolic acidemia. The newborn infant may not manifest acidemia in the first day or two of life, perhaps because of favorable blood tissue dissociation characteristics or tissue resistance factors. Inevitably, unless intracardiac mixing is improved by palliative or corrective intervention, severe hypoxemia results in advanced acidemia, hypoglycemia, hypothermia, and eventual death
- 61. TGA (VSD large) Good Mixing Presentation in this TGA group generally occurs in the latter half of the first month, with mild cyanosis and signs of heart failure resulting from pulmonary venous hypertension and myocardial failure. Tachycardia, tachypnea, important liver enlargement, and moist lung bases are present. The heart is more active and usually larger than in the poor-mixing group. A large VSD is associated with a moderate-intensity pansystolic murmur along the lower left sternal edge that may not be present initially. There is usually an apical middiastolic murmur or gallop rhythm and narrow splitting of the second heart sound with accentuation of the pulmonary component. With a large PDA, a continuous murmur, bounding pulses, and an apical middiastolic murmur are present in less than half the patients, even when the ventricular septum is intact.
- 62. TGA ( VSD and LVOTO) Large VSD with LVOTO is the least common of the three TGA groups. LVOTO is associated with a decreased Qp and poor mixing, but pulmonary venous hypertension and associated symptoms and signs do not develop because of lack of increase in Qp. Heart failure is therefore not present. cyanosis is severe from birth. Chest radiography shows a near normal-sized heart with normal or ischemic lung fields. ECG shows biventricular hypertrophy.
- 63. TGA (VSD and PVOD) Maybe mildly tachypnea, cyanotic at birth secondary increase in cyanosis with increasing haematocrit Symptoms of CHF improves
- 64. TGA with or without VSD + PDA Mild cynosis in initial few weeks of life. By end of first week symptoms of CHF starts. Sudden deterioration of clinical status with severe cynosis indicates spontaneous PDA closure
- 65. Reverse differential cyanosis, that is, cyanosis of the upper body greater than that of the lower body, is rare and indicates the presence of TGA with a PDA and pulmonary artery-to-aorta shunting prompt echocardiographic examination is clearly indicated for any cyanotic neonate with suspected congenital heart malformation Risk for necrotizing enterocolitis may be increased in neonates with TGA and a large PDA. Mesenteric circulation may be at risk because of (a) retrograde diastolic flow in the descending aorta producing a steal phenomenon, (b) Decreased oxygen delivery, (c) Cardiac catheterization/angiography and umbilical artery catheterization in some cases.
- 66. ARTERIAL PULSE and JUGULAR VENOUS PULSE • the bounding pulses,the scalp varices, and the warm extremities to the large volume of highly unsaturated blood recirculating in the hyperkinetic low-resistance systemic vascular bed • Diminished femoral pulses call attention to coexisting coarctation of the aorta with anterior and rightward deviation of the infundibular septum(subaortic stenosis). • jugular venous pulse is elevated
- 67. PALPATION • A loud palpable second heart sound at the left base originates in the aortic valve because the transposed aorta is anterior • A left ventricular impulse is not identified in neonates because ejection is at a low systolic pressure.
- 68. AUSCULTATION • Pulmonary ejection sounds • Midsystolic flow murmurs originate in the transposed anterior aorta • holosystolic murmur of ventricular septal defect • The murmur of a nonrestrictive patent ductus arteriosus is confined to systole • Mid-diastolic murmurs are generated across the mitral valve when pulmonary blood flow is increased • A loud and single second heart sound is the aortic because the aorta is anterior
- 69. ELECTROCARDIOGRAM •Tall peaked right atrial P waves soon emerge because mean right atrial pressure is increased •Right axis deviation is most striking when an atrial septal defect occurs with pure right ventricular hypertrophy •Biventricular hypertrophy is evidence of a nonrestrictive ventricular septal defect with low pulmonary vascular resistance
- 70. X Ray • In the neonate with TGA/IVS, the diagnostic triad includes (a) oval or egg-shaped cardiac silhouette with narrow superior mediastinum (b) mild cardiomegaly (c) increased pulmonary vascular markings.
- 71. • right border of the egg consists of the right atrium • the convex left border is the left ventricle. • In the lateral projection, the heart assumes a circular appearance because an enlarged right ventricle merges with the anterior aorta, and an enlarged left ventricle merges with the dilated posterior left atrium
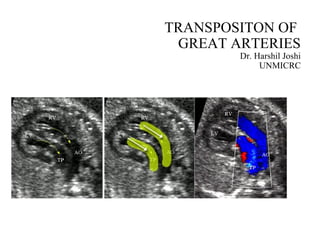
- 72. Echocadography • Fetal Echocardiography • Postnatal Echocardiography
- 73. Cath Study • Those hemodynamically unstable and rquire BAS. • Where more physiologic and anatomic data is required concerning coronary artery, the VSD, degree of LVOTO. • Presence of other complex cardiac anomalies like CoA, interrupted aortic arch. • To quantify pulmonary vascular resistance in patients of TGA with VSD
- 74. Medical management • Important in patients with TGA with IVS. • Focuses on hemodynamic stabilization and correction of physiological abbarations caused by cyanosis and poor perfusion. • Correction of acid base balance, maintainance of normothermia, prevention of hypoglycemia. • PGE1 infusion to maintain patencty of ductus to provide mixing of saturated and desaturated blood. • BAS
- 75. Aims of surgery • To make the parallel circulations into series. So that oxygenated blood goes to aorta and deoxygenated blood goes to pulmonary trunk. • Correction of other cardiac anomalies like VSD, PDA, TR, AORTIC OBSTRUCTION, LVOTO. • To provide a near normal functional status to patients.
- 76. Treatment- Palliative • balloon atrial septostomy • surgical septectomy • partial venous correction • pulmonary artery banding • systemic-to-pulmonary shunts
- 77. Surgery for TGA/IVS or TGA/VSD without Associated Outflow Tract Obstruction • Physiologic Correction (Atrial Switch) • Senning repair-- the atrial baffle is fashioned in situ using tissue from the right atrial wall and interatrial septum • Mustard operation--- after resection of most of the atrial septum, the baffle is made from autologous pericardial tissue or (rarely) synthetic material
- 78. Complications of atrial switch • caval and pulmonary venous obstructions • residual intra-atrial shunts • Atrial and ventricular arrythmias • Tricupid valve regurgitation • Right ventricular failure
- 79. Explanations for postatrial repair subnormal right (systemic) ventricular function • Right ventricular myocardial fiber arrangements is not optimum and mismatch between right ventricular coronary blood supply and systemic ventricular work demand. • The left ventricular free wall is composed predominantly of stratum compactum, whereas the right ventricular free wall consists predominantly of stratum spongiosum. • Transposed ventricular pressure relationships force the ventricular septum to bulge posterior-leftward and to present a concave septal surface during contraction. • the tricuspid valve is supported by relatively small papillary muscles in comparison with the mitral valve, and this, in conjunction with the abnormal concave right ventricular septal surface, may result in TR.
- 80. Anatomic Correction (Arterial Switch) • The great arteries are transected in a manner that allows eventual reanastomosis of the distal aortic segment to the proximal pulmonary artery (neoaortic root). Transfer of the coronary arteries to this pulmonary segment is facilitated by their excision from the aortic sinus with a cuff of adjacent aortic wall. The proximal aortic segment (neopulmonary root) may be connected to the distal pulmonary artery segment by an end-to-end anastomosis using the innovative maneuver of Lecompte
- 81. • Anatomic variants that may impact operative mortality include • (a) an intramural course of a coronary artery • (b) a retropulmonary course of the left coronary artery • (c) multiple VSDs • (d) coexisting abnormalities of the aortic arch • (e) straddling AV valves
- 82. Surgery for Transposition of the Great Arteries with Low Left Ventricular Pressure • Pulmonary artery banding has been used to increase left ventricular afterload and stimulate hypertrophy • Intraoperative TEE is useful in guiding placement of the PAB. The band is tightened enough to flatten the intraventricular septum by shifting it toward the RV • When this technique is used, left ventricular function may be extremely impaired following banding; therefore, systemic-to- pulmonary artery shunt is frequently placed to ensure adequate pulmonary blood flow • The interval period between banding and correction is frequently characterized by a low output syndrome, most likely resulting from a combination of acute (fixed) right ventricular volume overload from the shunt and acute (transient) left ventricular dysfunction from the pulmonary artery band
- 83. Left Ventricular Preparation • direct pressure measurements in the left ventricle or the ratio of left- to-right ventricular pressure may not be predictive of the capability of the left ventricle to perform systemic work • in a neonate with immediate closure of the ductus and a prompt fall in pulmonary vascular resistance, the left ventricular systolic pressure can fall to less than half the systemic levels (or 25 to 30 mm Hg) as soon as 4 or 5 days after birth • A 6-week-old patient whose ductus has only recently closed will more likely have a prepared left ventricle than a 6-week-old patient whose ductus closed directly after birth
- 84. Empiric criteria to determine adequate left ventricular preparation • an absolute left ventricular systolic pressure that is appropriate for age • a left ventricular pressure at cardiac catheterization that is 70% systemic levels (left to right ventricular ratio >0.7) • left ventricular muscle mass that is within the normal range for body surface area
- 85. other innovative approaches • percutaneously adjustable band • partial balloon occlusion of the main pulmonary artery with a percutaneously placed balloon-tipped catheter • systemic-to-pulmonary artery shunting alone • primary arterial switch with left ventricular assist in the perioperative period
- 86. Anatomic Correction without Coronary Translocation • Damus, Kaye, and Stansel(DKS)
- 87. Surgery for Transposition of the Great Arteries with Associated Left Ventricular Outflow Tract Obstruction • dynamic LVOTO is reduced or eliminated in older infants with TGA/IVS following pulmonary artery banding • Transpulmonary or transmitral resection has been performed for severe fixed obstruction caused by a short, discrete fibromuscular subvalvar shelf • the Rastelli operation • REV(Reparation a L'etage Ventriculaire) by Lecompte
- 88. Surgery for Right Ventricular Failure Following Physiologic Correction • tricuspid valvuloplasty/replacement • cardiac transplantation • anatomic correction • Damus,Kaye,Stansel operation may be technically easier to perform, but it requires the use of a prosthetic conduit from the right ventricle to pulmonary arteries
- 89. Surgery for TGA/VSD and Pulmonary Vascular Obstructive Disease • Advanced pulmonary vascular disease characterized by calculated pulmonary vascular resistances >10 U or grade 4 (H-E) histologic changes is considered a contraindication to closure of the VSD • Palliative switch (either atrial or arterial) allows more effective pulmonary and systemic flows and a significantly improved systemic arterial oxygen saturation.
- 92. Initial palliative management • Most patient at admission are dehydrated, acidosis and cyanotic • Give hydration therapy with oxygen • Prostagalandin E1 therapy • Rashkind ballon septoplasty • Ventilation may require • Aggressive ventilation with high PEEP compromise PBF • High Fio2 may close PDA • Maintain SaO2 of 80-85%
- 93. Anesthetic Goals in Patients with Transposition of the Great Vessels • Maintain heart rate, contractility, and preload to maintain cardiac output. Decreases in cardiac output decrease systemic venous saturation with resultant fall in arterial saturation. • Maintain ductal patency with prostaglandin E1 (0.05-0.1 µg/kg/min) in ductal-dependent patients. • Avoid increases in PVR relative to SVR. Increases in PVR decrease pulmonary blood flow and reduce intercirculatory mixing. In patients with pulmonary vascular occlusive disease, ventilatory interventions should be used to reduce PVR • Reductions in SVR relative to PVR should be avoided. Decreased SVR increases recirculation of systemic venous blood and decreases arterial saturation.
- 94. Premeditation • rarely necessary • prostaglandin E1 infusion should be continued until cardiopulmonary bypass (CPB) • oral midazolam 0.5 to 1.0 mg/kg is a useful premedicant • preoperative intravenous hydration
- 95. Monitoring • blood pressure cuff, ECG, pulse oximeter, end-tidal carbon dioxide monitor, and precordial stethoscope • intraarterial catheter • central venous catheters • Nasopharyngeal, tympanic, and rectal temperature • transesophageal echocardiography
- 96. Induction • opioids alone in high doses (25-100 µg/kg fentanyl or 2.5-10 µg/kg sufentanil) • provide hemodynamic stability, do not depress the myocardium, and blunt reactive pulmonary hypertension • low-to-moderate doses (5-25 µg/kg fentanyl or 0.5-2.5 µg/kg sufentanil) in combination with an inhalation agent • avoid bradycardia • Ketamine does not increase PVR as long as normocarbia is maintained and hypoxemia avoided • PCO2 ranging from 25 to 35 mmHg (3-5 kPa) (38), and pH ranging from 7.50 to 7.56 (39) effectively reduce PVR in infants • Hypercarbia, acidosis, and hypoxemia should be avoided • Low PEEP and Low FiO2
- 97. Intraoperative Management • Priming with Blood and Albumin • Mainatian ACT • Mainatain haematocrit more than 25% • DHCA management • Blood Cardioplegia • CUF and MUF should be used • Phenoxybenzamine can be used • Milrinone should be loaded
- 98. Complication at off CPB • SVC and IVC obstruction • Pulmonary vein obstruction • TEE is extremely useful for detecting venous obstruction • Arrhythmias immediately following the atrial switch procedures may be problematic • Coronary hypoperfusion and Myocardial ischemia • Mainatin high perfusion pressure on CPB • Inotropic support of the LV and afterload reduction may be necessary to terminate CPB
- 99. Post CPB • overzealous volume infusion can result in LV distention and LA hypertension • LA hypertension produces elevations in PA pressure and distention of the PA • distention of the PA may compress or place tension on the coronary ostia • Weaned of bypass at 36 temp • PD catheter and chest open decision depending on situation
- 100. Post op Care • Depends on type of operation • Ventilate for 24 hour…. Early extubation can be done in experience center • Mainatain normo or hypocarbia with min FiO2 • Narcotic analgesia • Post op Echo • ST segment monitoring • U/O and Infection • Inhaled NO to decrease PA pressure • Milrinone or levosimendan- decrease afterload and inotropic • Avoid high dose adrenalin– alpha effect
- 101. • If LA line present fluid accordingly • left atrial (or pulmonary artery diastolic) pressure should remain low, less than about 12 mmHg • Accept mean BP of 35 mm hg in neonate • In ASO maintain coronary perfusion • Add noradrenalin to conteract milrinone induced very low afterload • Lactate level and mix venous saturtation measure • Strat RT feed or NG drip as early as possible • Negative balance at the end of day • Never give bolus fluid.. Low CVP is not indication for Fluid • Echo guided fluid replacement
- 102. • In Atrial Switch • Positive end-expiratory pressure (PEEP) is not used because it tends to obstruct the SVC • Infants are nursed in a slightly head-up position • Atrial pressures are kept as low as is compatible with an adequate cardiac output
- 103. Complication • Low CO • Coronary Ischemia • Pulmonary hypertensive crisis • Mesenteric Ischemia • Convulsion • Renal Failure • Sepsis
- 104. THANK YOU
