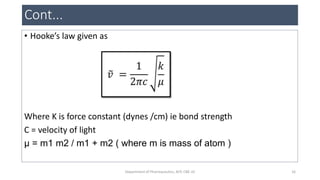
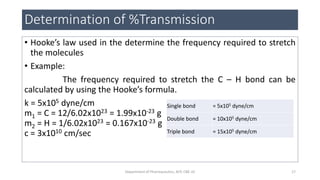
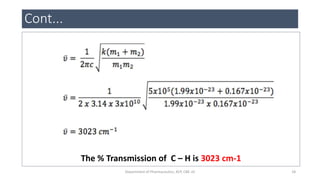
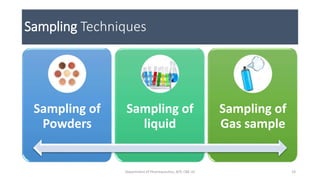
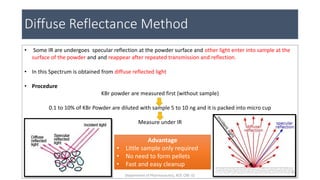
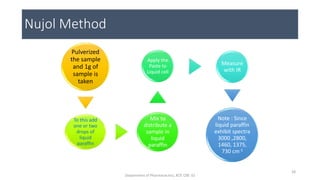
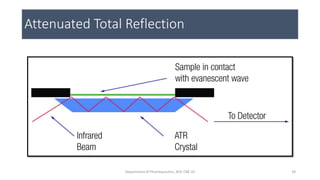
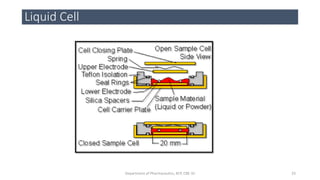
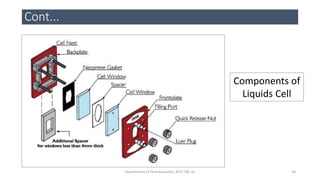
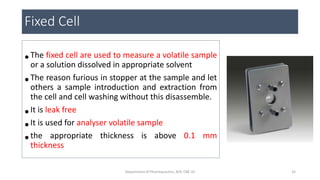
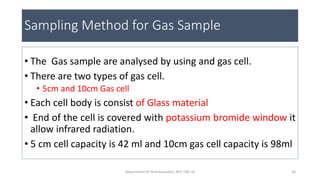
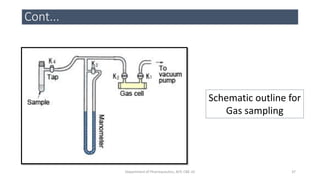
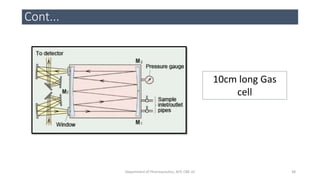
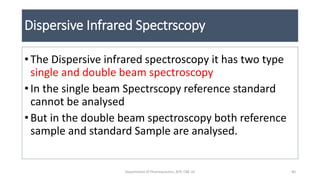
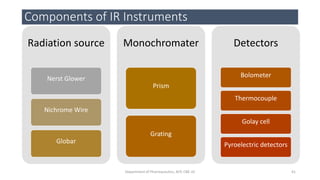
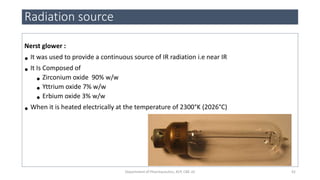
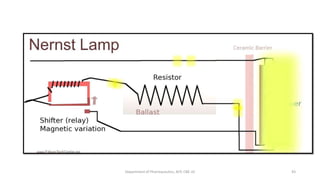
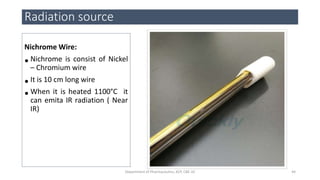
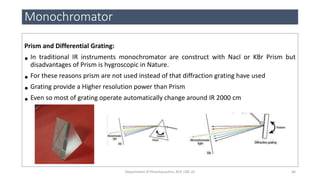
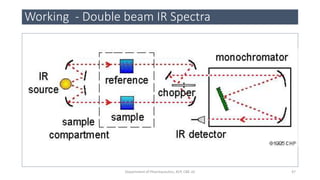
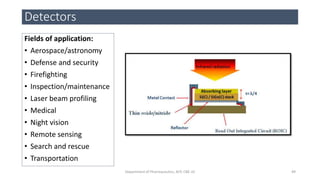
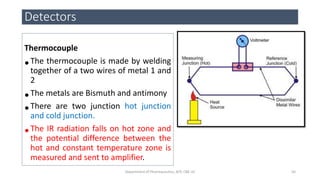
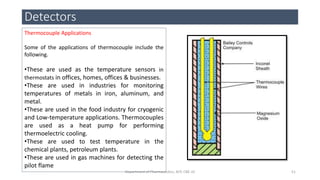
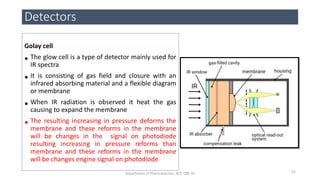
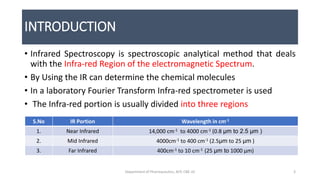
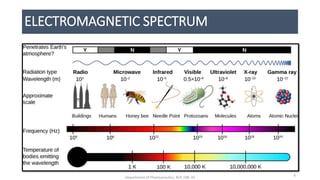
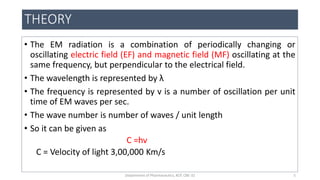
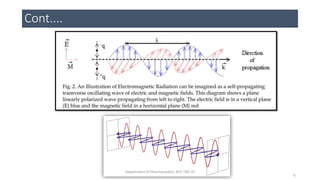
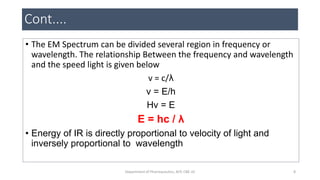
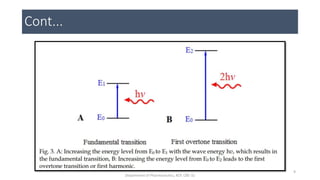
This document provides an overview of infrared spectroscopy. It discusses the electromagnetic spectrum and how infrared spectroscopy uses infrared light to analyze chemical bonds in molecules based on their vibrational and rotational frequencies. Different sampling techniques are described for analyzing powders, liquids, and gases. Key points covered include the principles of infrared absorption, molecular vibrations, Hooke's law application to frequency determination, and methods for preparing samples like KBr pellets and diffuse reflectance.


![Molecular Vibrational
• The IR energy absorption corresponds to specific modes ; It
corresponding to combination of atomic movements.
• Vibrational force are two type
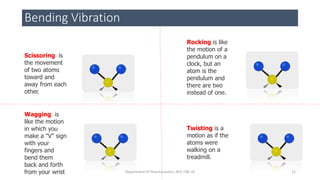
• BENDING VIBRATIONAL FORCE [change in bond angles]
• Scissoring
• Wagging
• Rocking
• Twisting
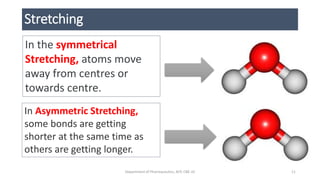
• STRETCHING VIBRATIONAL FORCE [Change in bond length]
• Symmetrical & Asymmetrical
• It corresponds to vibrational and rotational force
10Department of Pharmaceutics, KCP, CBE-32](https://image.slidesharecdn.com/infraredspectroscopy-190508060059/85/Infrared-spectroscopy-10-320.jpg)