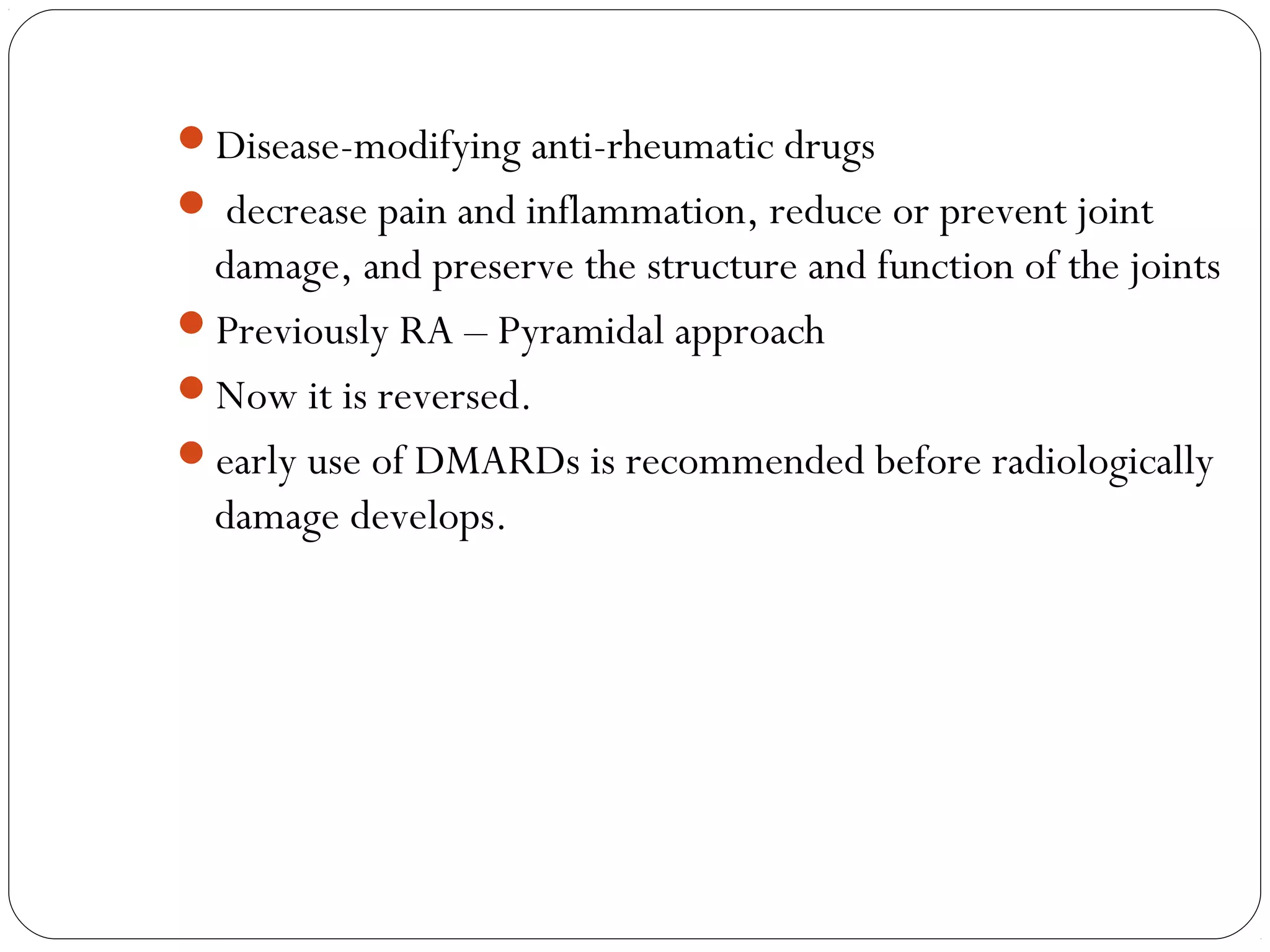
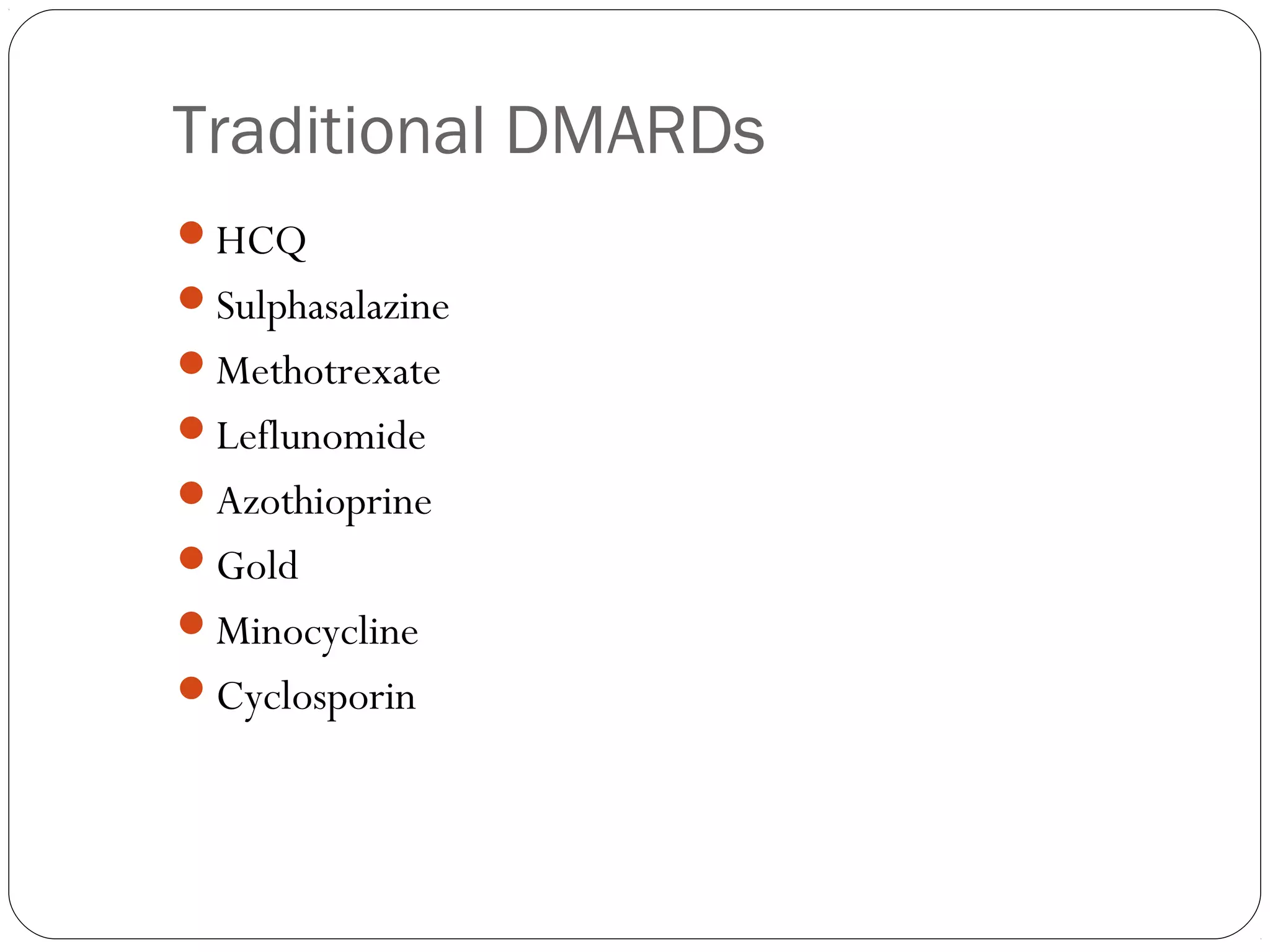
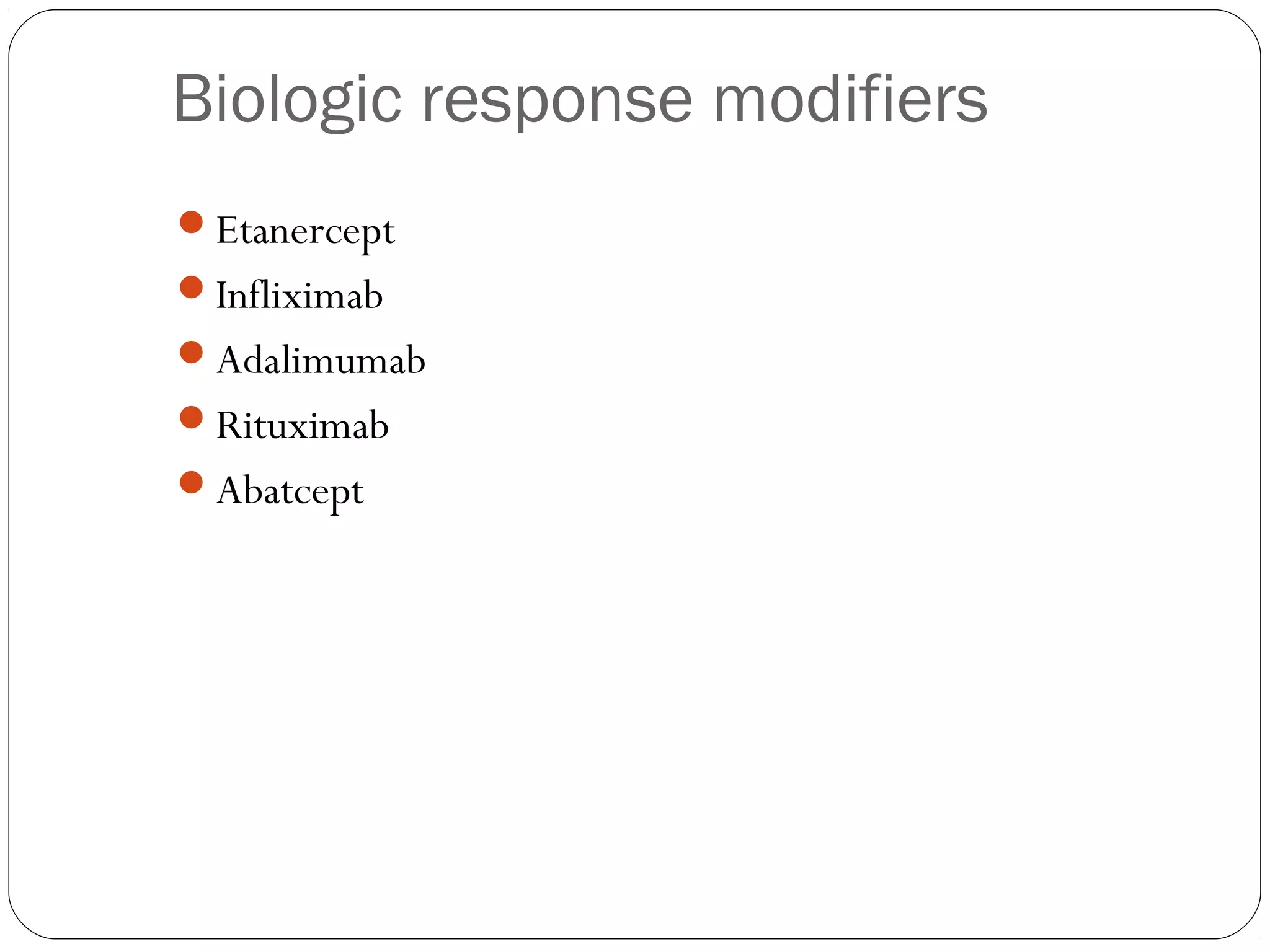
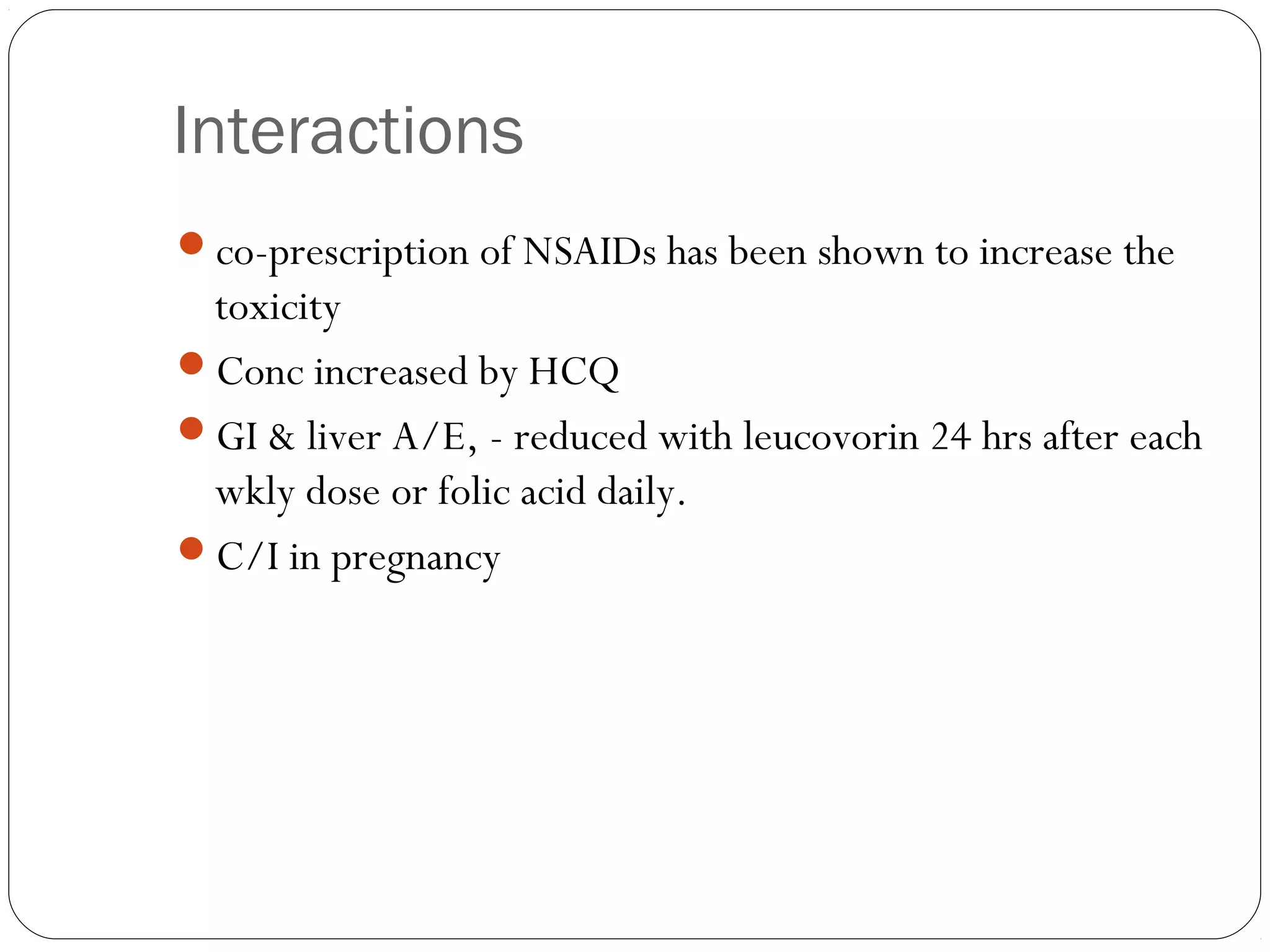
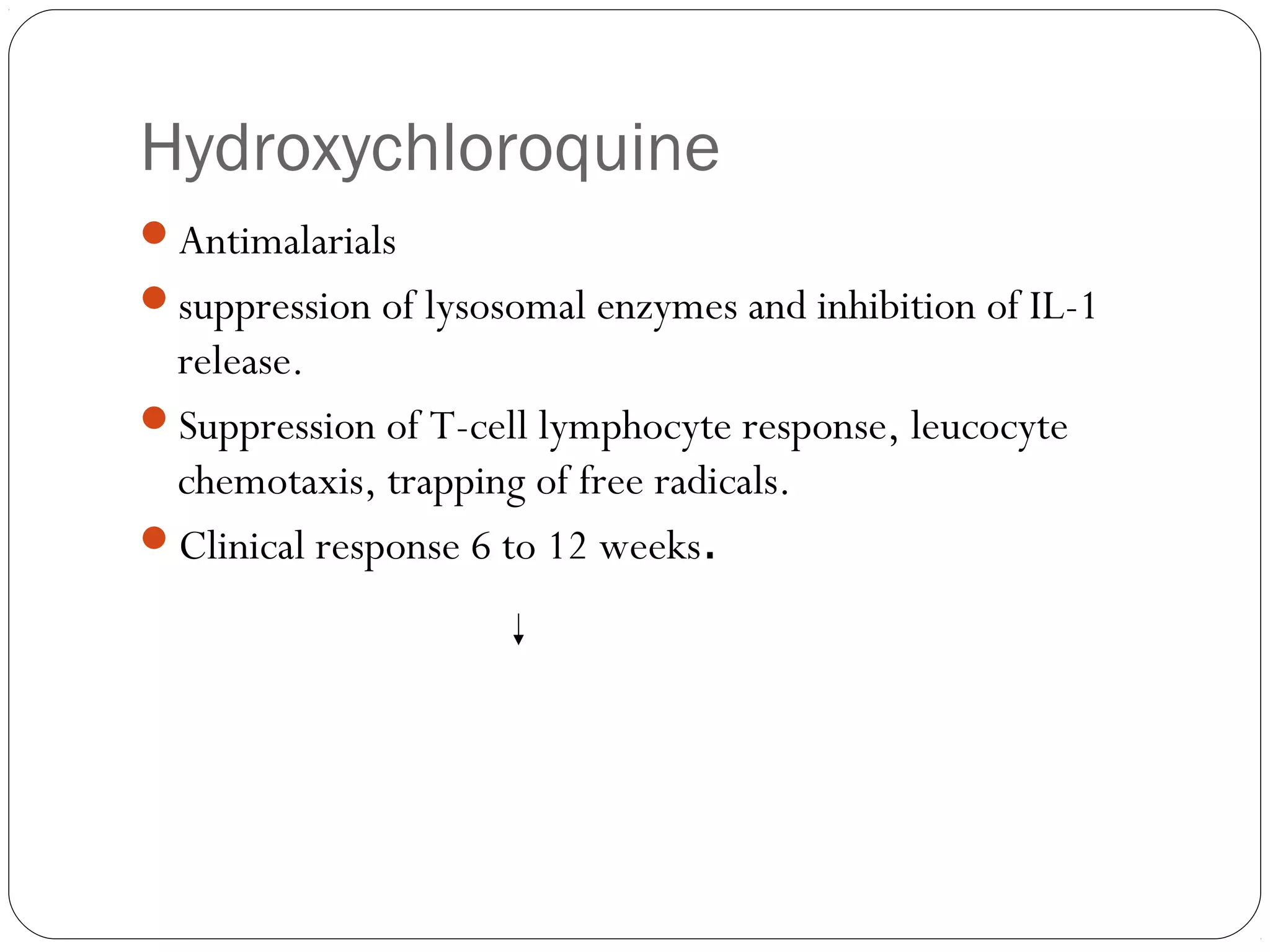
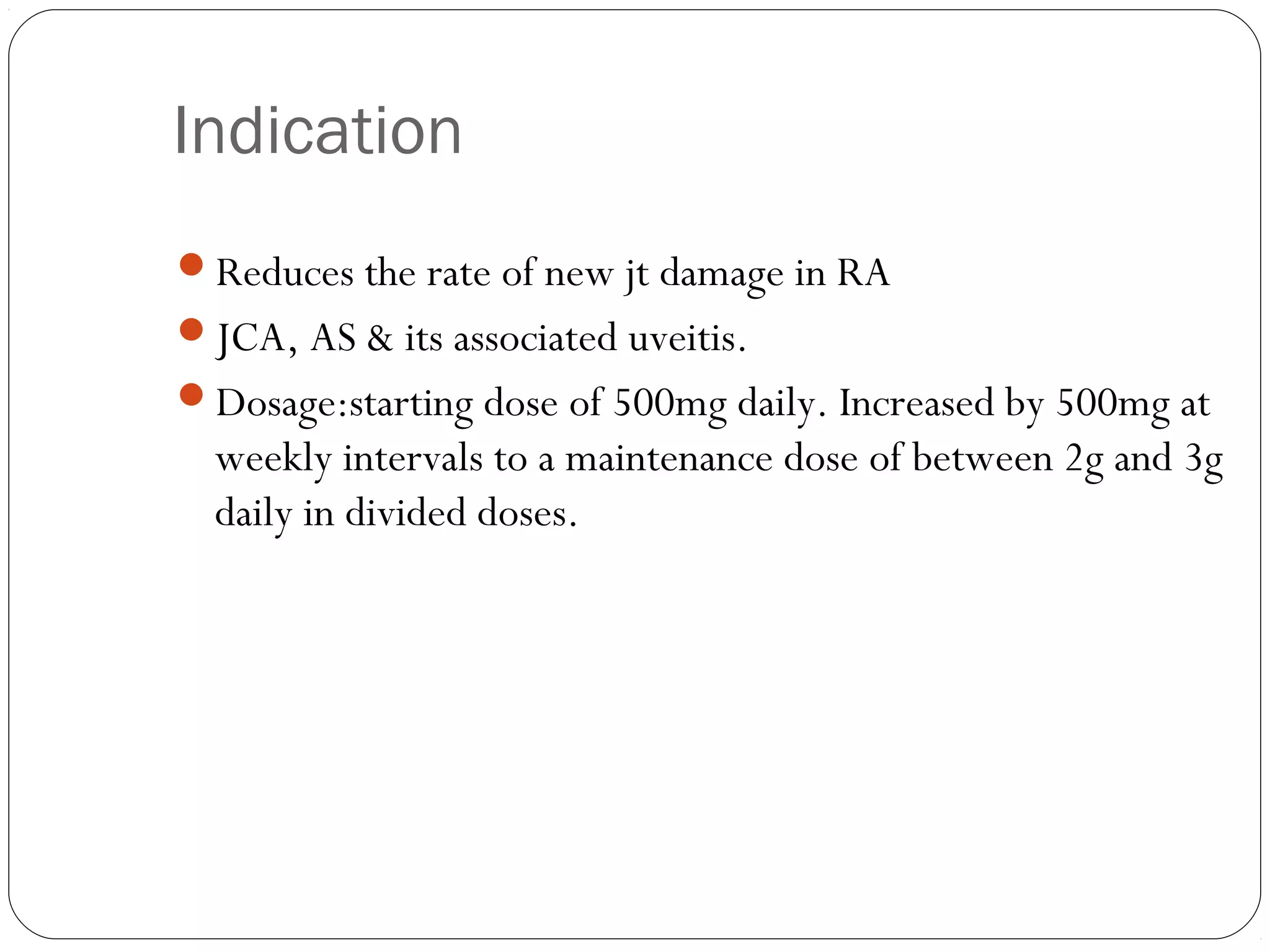
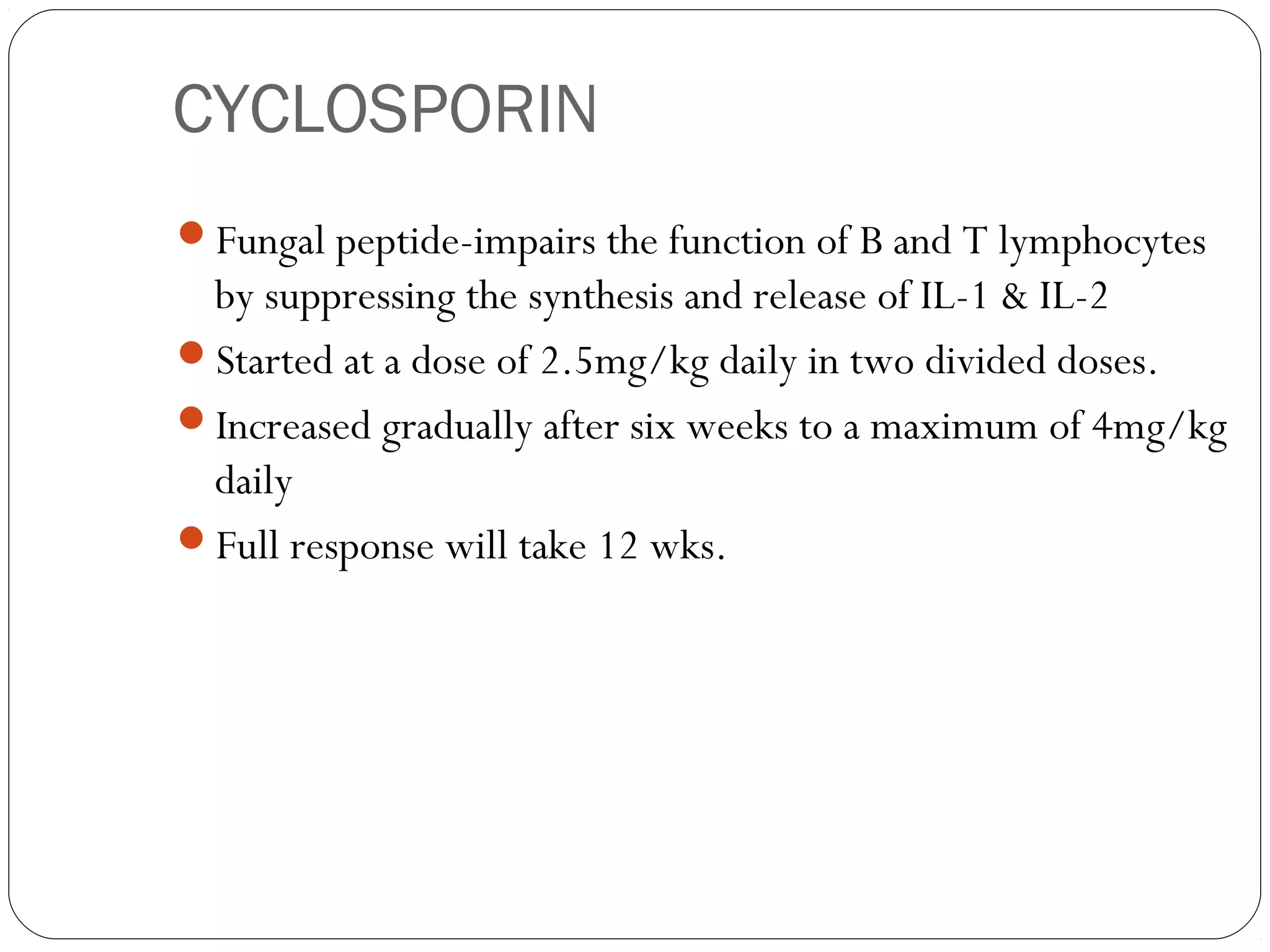
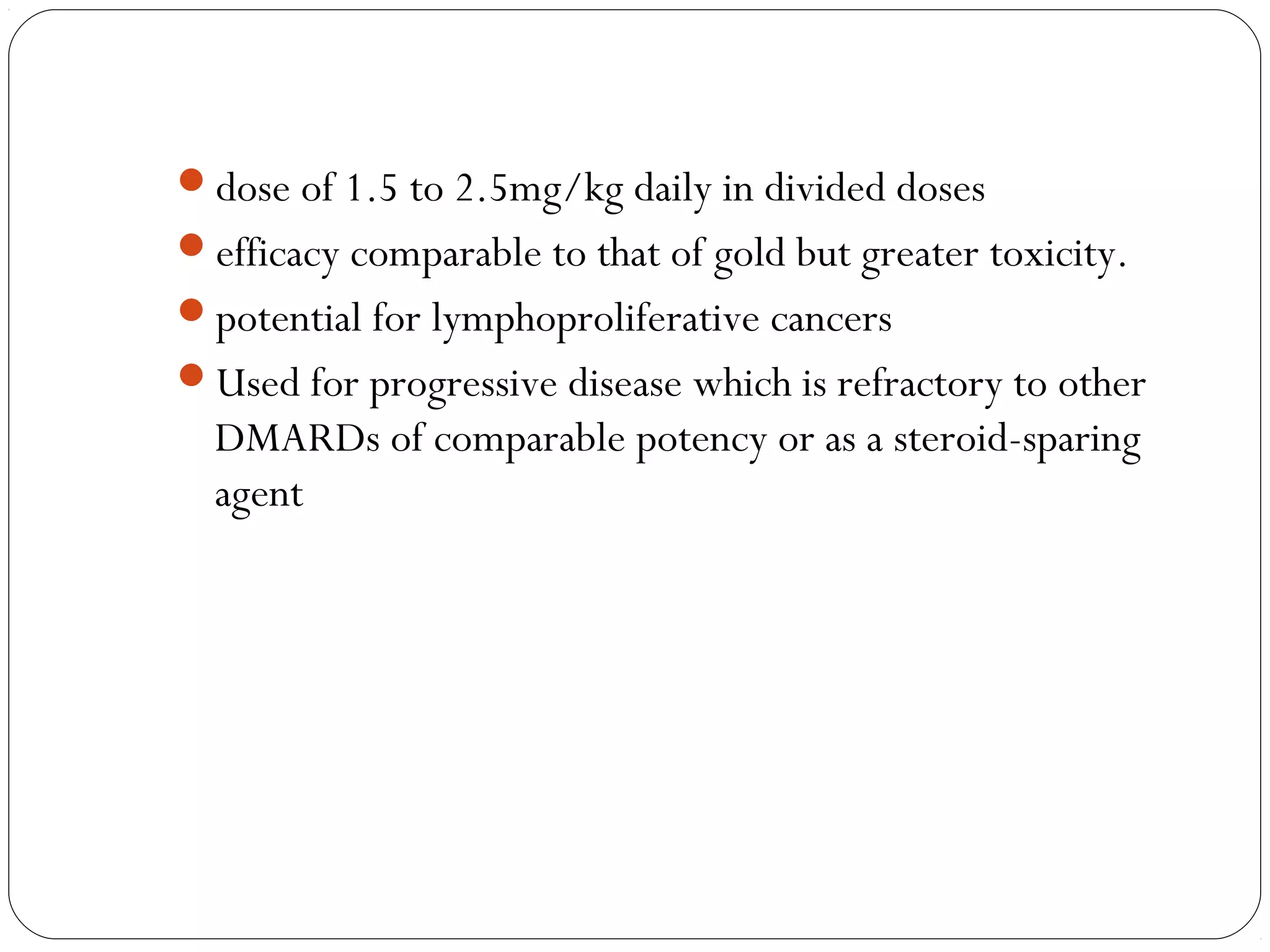
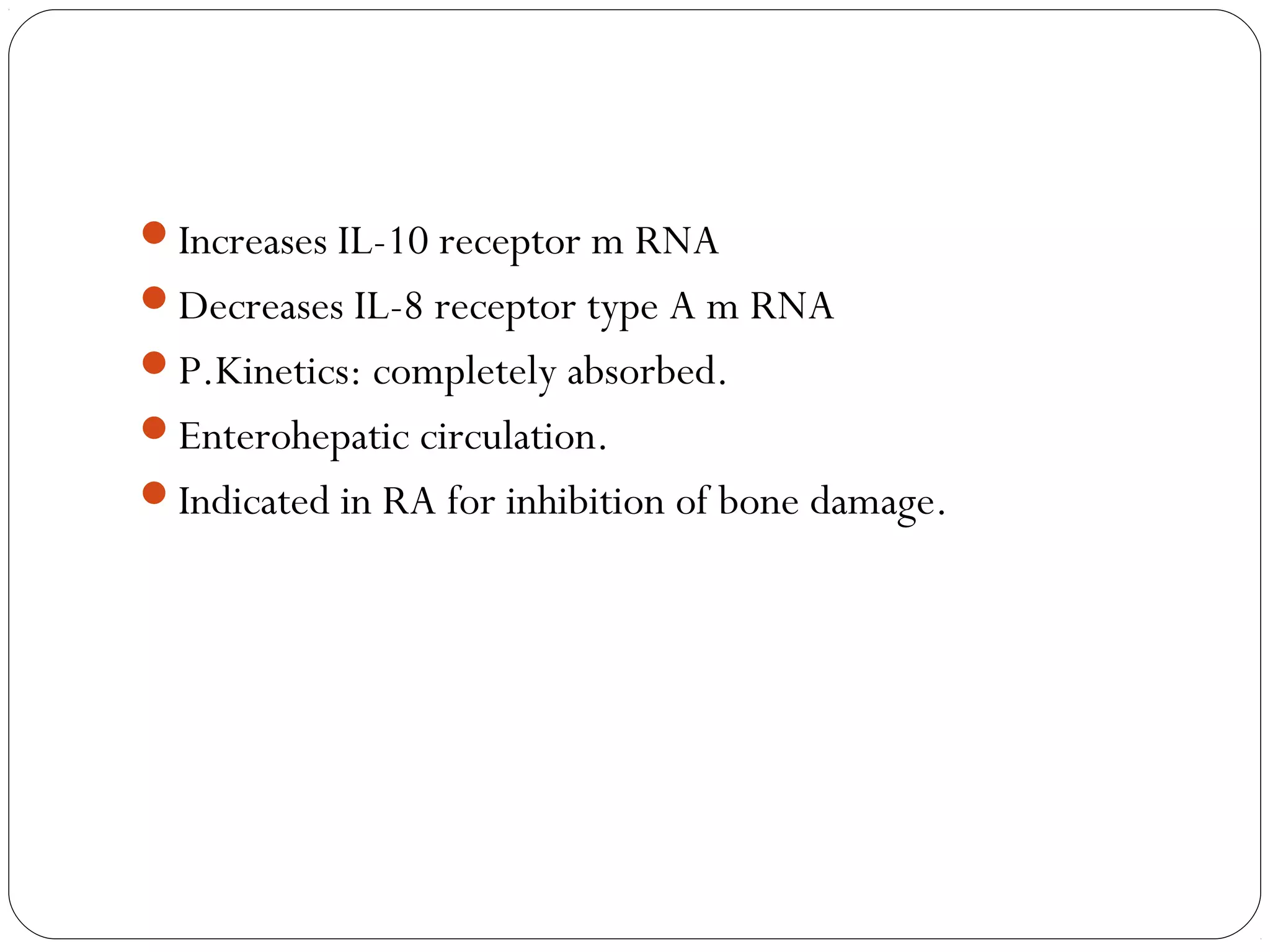
DMARDs are used to decrease pain, inflammation, and prevent joint damage in rheumatoid arthritis. They work by limiting inflammation early in the disease course before structural damage occurs. The traditional DMARDs include methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide, while biologic DMARDs target cytokines like TNF-α. Methotrexate is usually the first choice due to its efficacy and safety profile. DMARD therapy should be started aggressively within 3 months of diagnosis to prevent long-term disability. Combination therapy and treatment adherence are important to control symptoms and progression of rheumatoid arthritis.




![METHOTREXATE
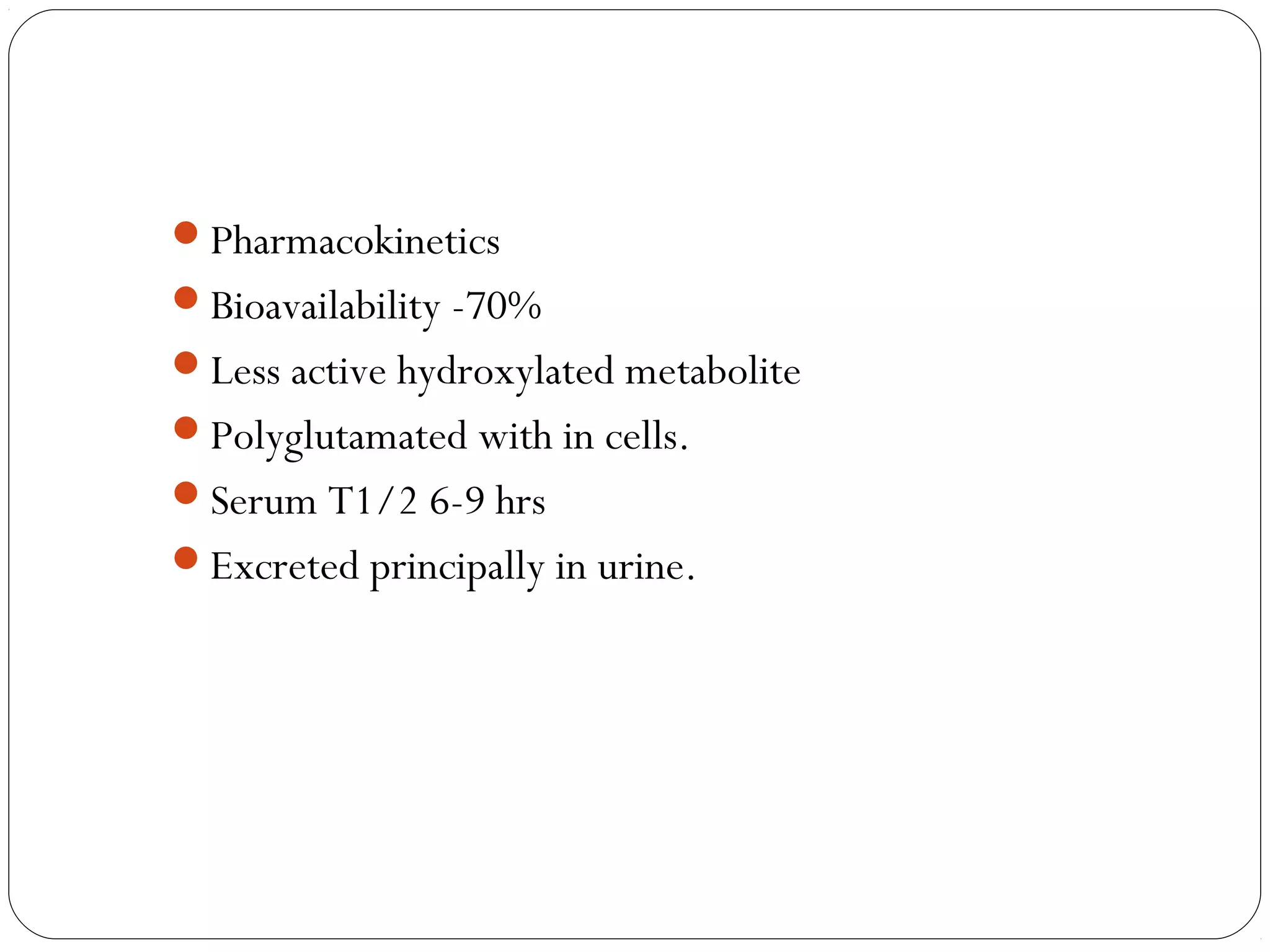
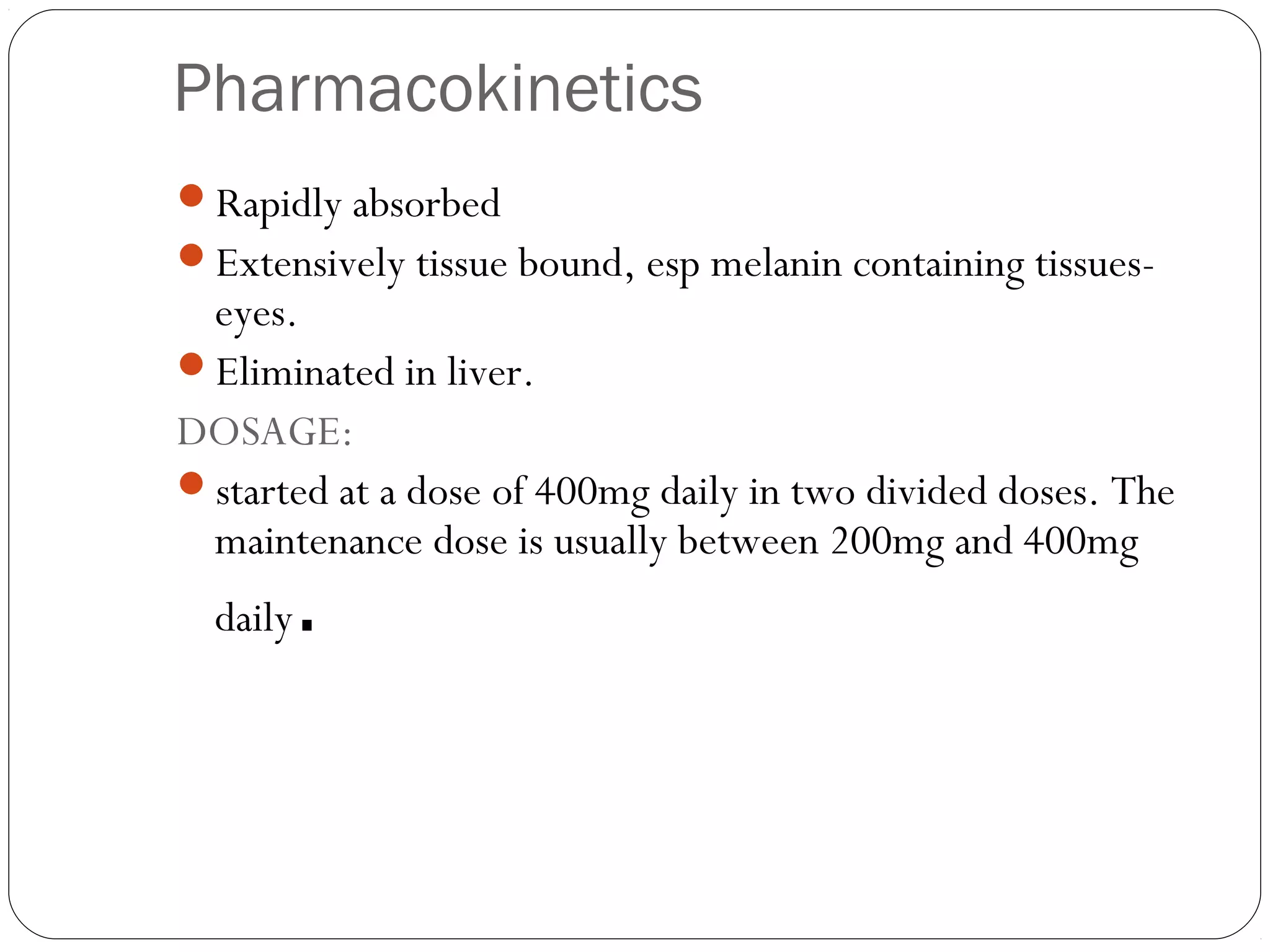
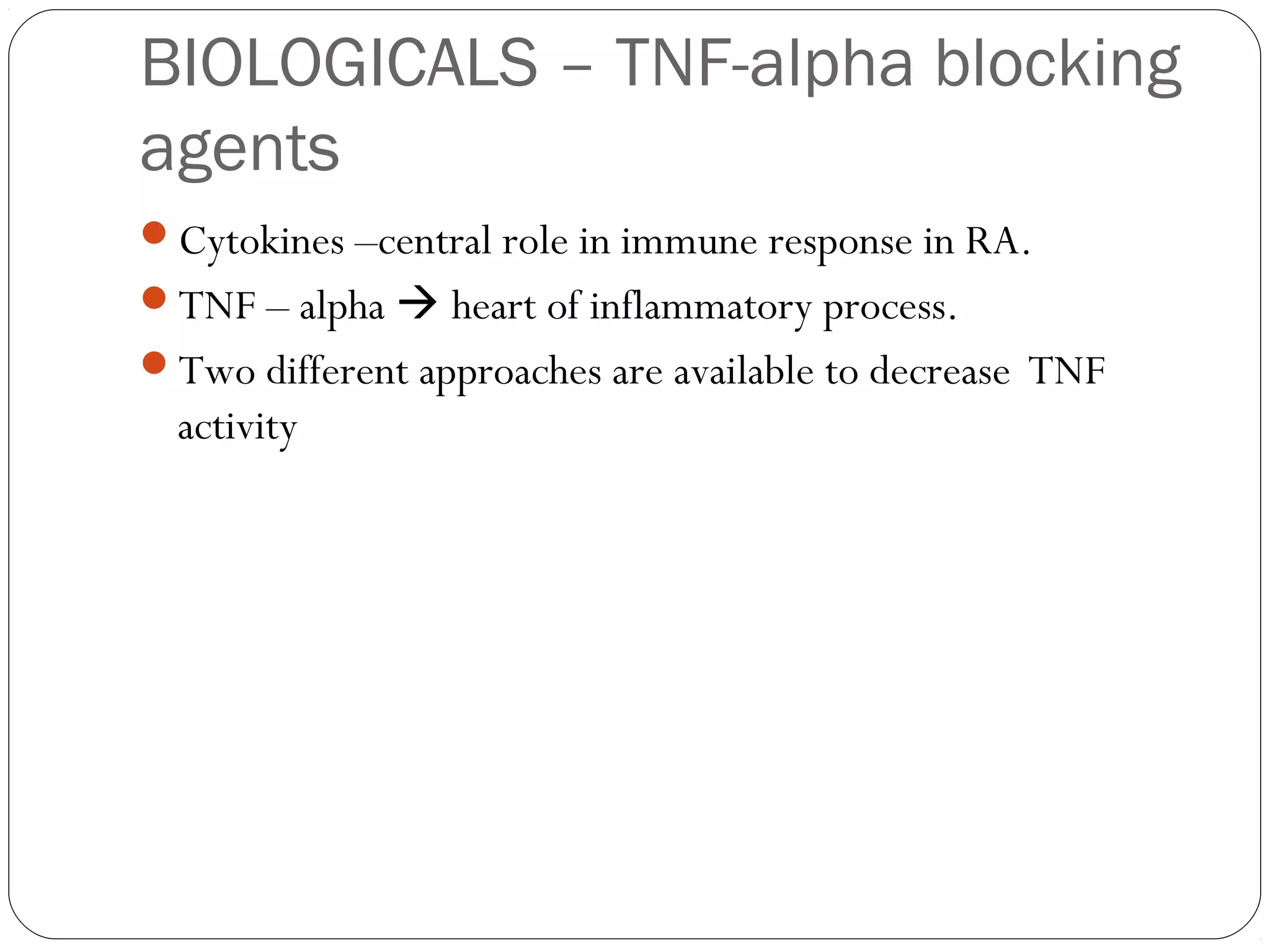
DMARD of choice.
MOA: Inhibition of [AICAR] aminoimidazolecarboxamide
transformylase & thymidylate synthetase.
decrease the secretion of pro-inflammatory cytokines, such as
TNF, while increasing the secretion of the inhibitory cytokine IL-
10.
Decreases the rate of appearance of new erosions](https://image.slidesharecdn.com/dmards-130422051053-phpapp01/75/Dmards-8-2048.jpg)


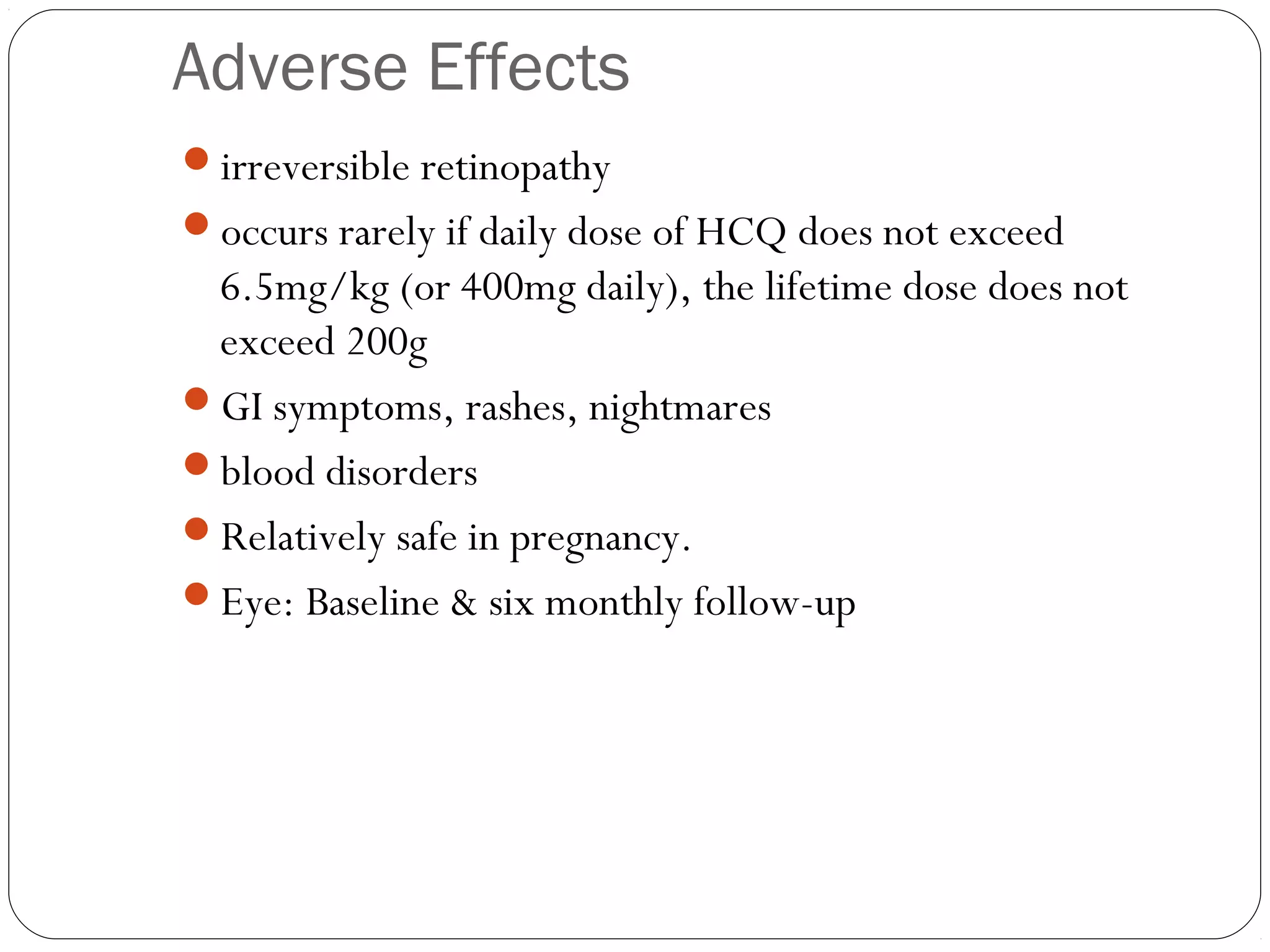
![Indications
RA
Not very effective. [bone damage]
Restricted to patients with mild, non-erosive disease or to
those in whom more powerful DMARD therapy is felt to be
too risky
used in combination with other agents](https://image.slidesharecdn.com/dmards-130422051053-phpapp01/75/Dmards-16-2048.jpg)


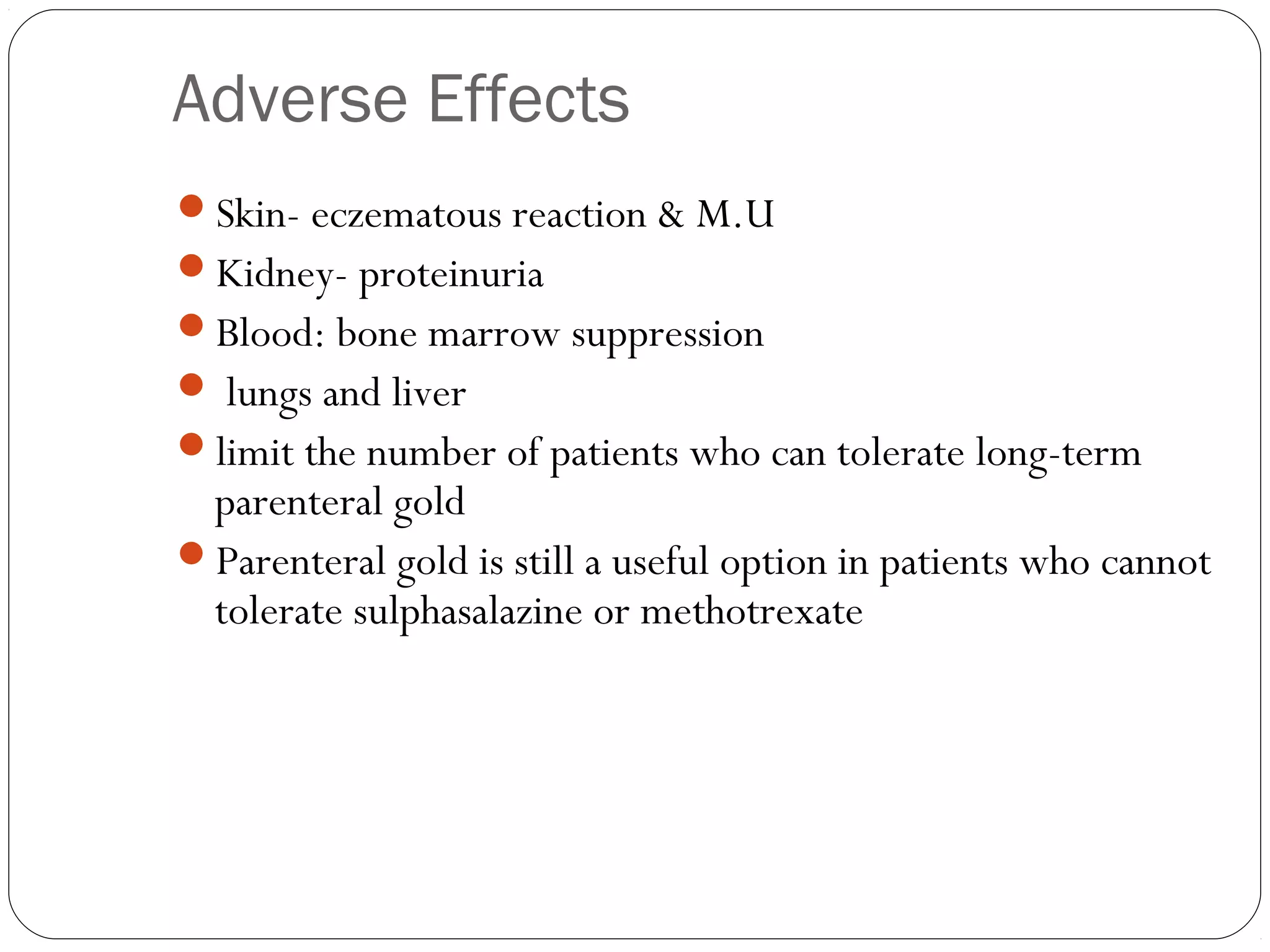










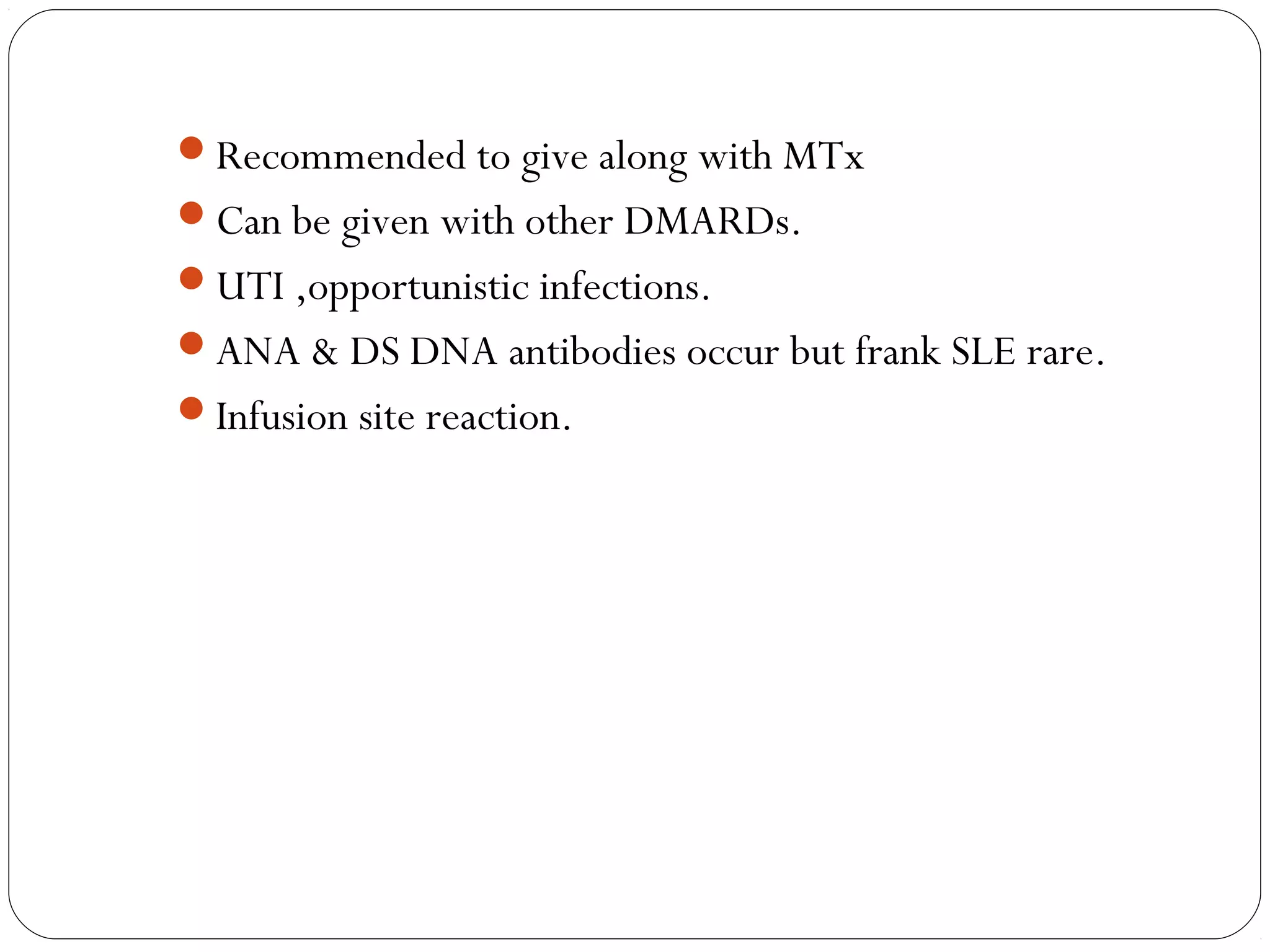
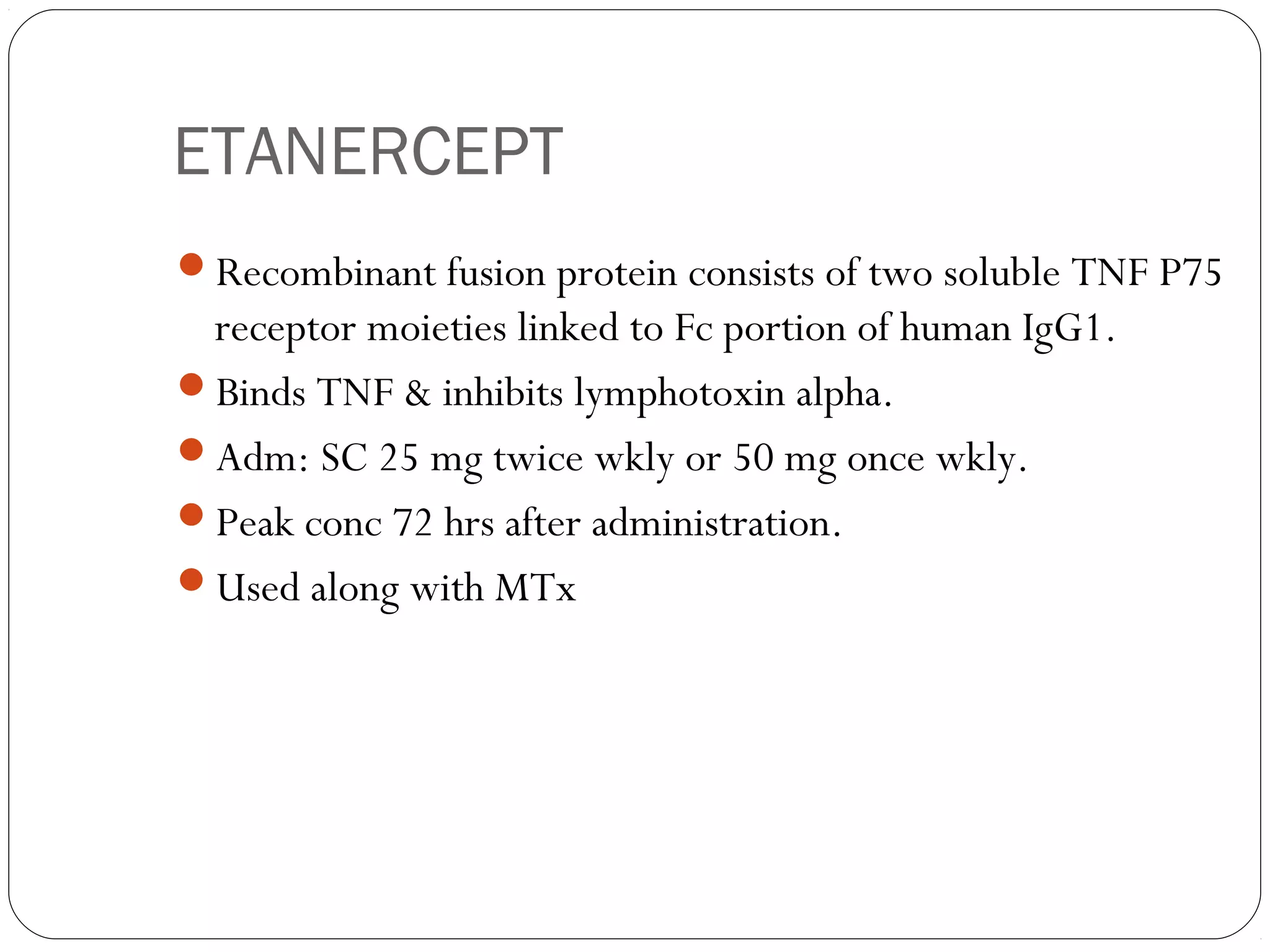
![INFLIXIMAB
Chimeric monoclonal antibody [25% mouse, 75% human]
Binds with both soluble & membrane bound TNF
IV infusions 3-5 mg/kg every 8 wks.
Antichimeric AB – 62% pts
Concurrent MTx adm, reduces.](https://image.slidesharecdn.com/dmards-130422051053-phpapp01/75/Dmards-37-2048.jpg)


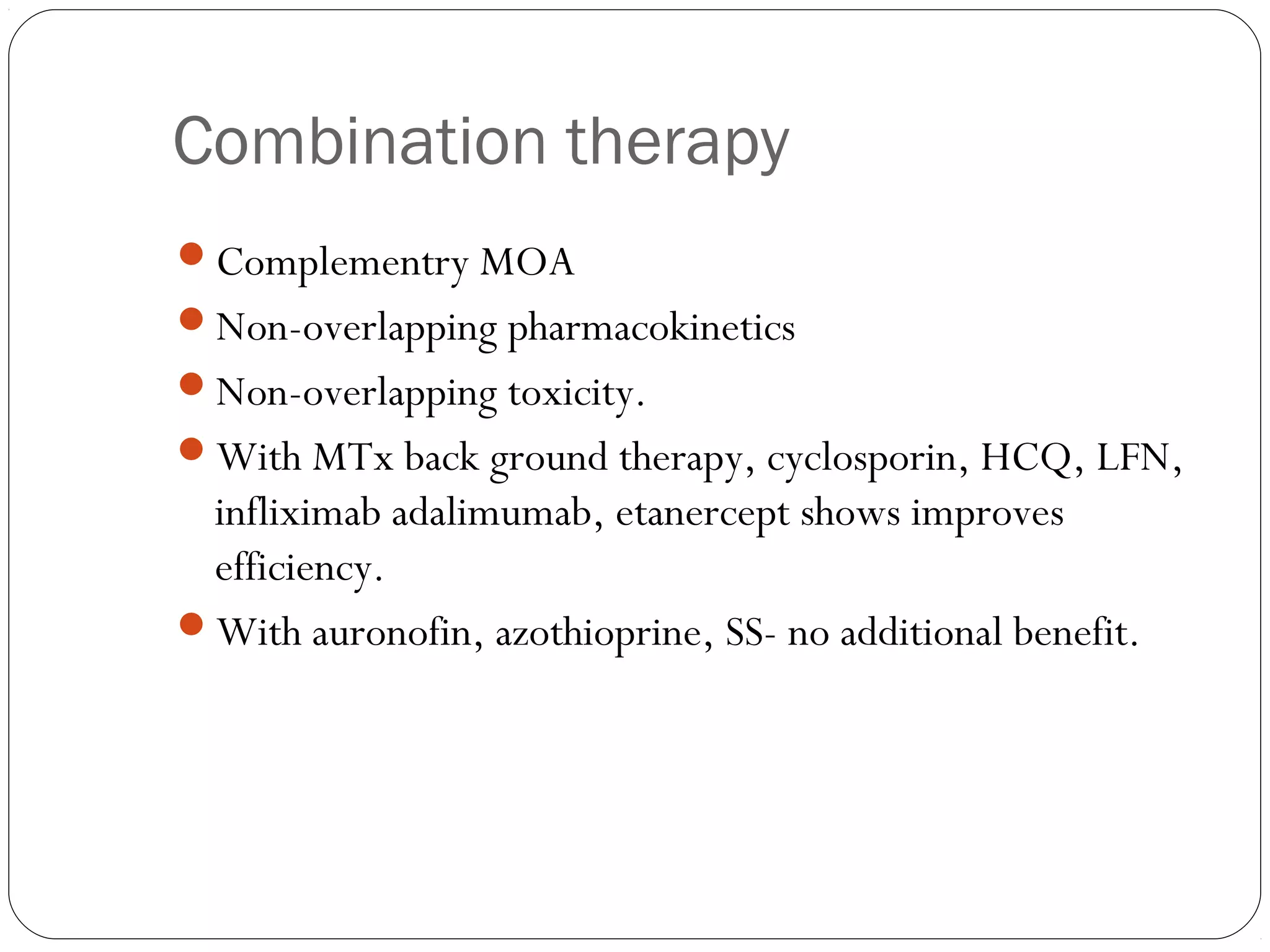
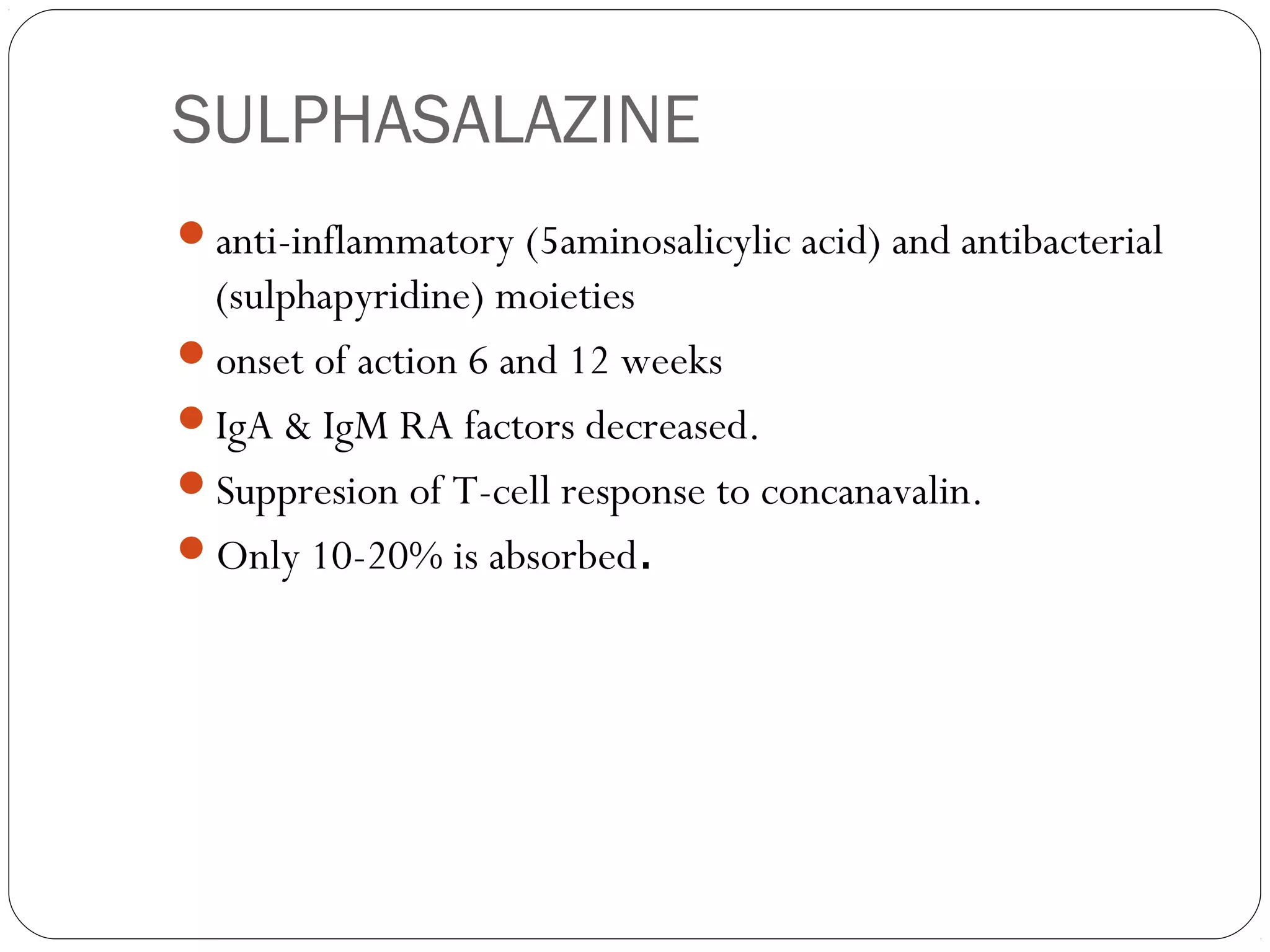
![Triple drug regimen: MTx, SS, HCQ.
Disadv: more toxicity [mostly not occurs]
C.T is becoming a rule for those not responding to
monotherapy.](https://image.slidesharecdn.com/dmards-130422051053-phpapp01/75/Dmards-43-2048.jpg)