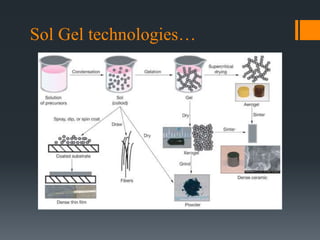
This document discusses various powder production techniques for ceramics. It begins by introducing ceramics and their properties. It then covers classification of ceramics and various raw materials used. The main powder production methods discussed are mechanical (milling), chemical, physical (freeze drying, spray drying, sol-gel) and some commercial examples. Ball milling, attrition milling, freeze drying process and applications, spray drying process and applications are described in detail. Advantages and disadvantages of freeze drying and spray drying are also provided.