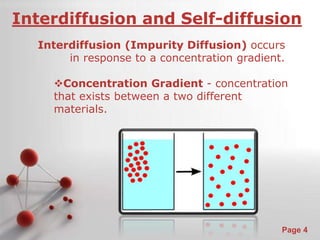
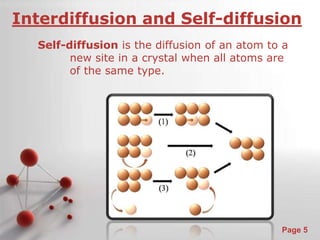
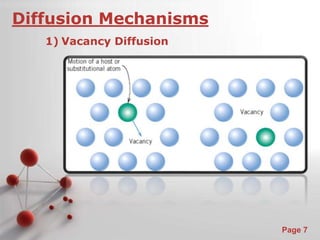
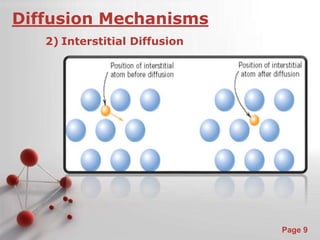
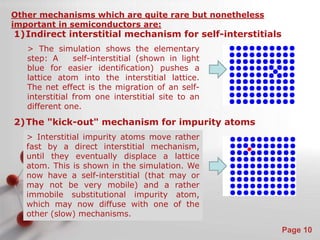
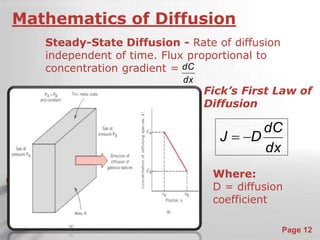
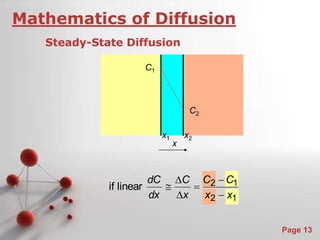
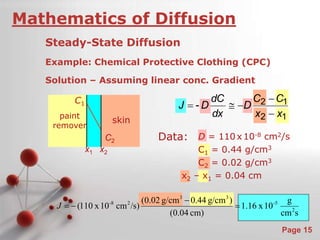
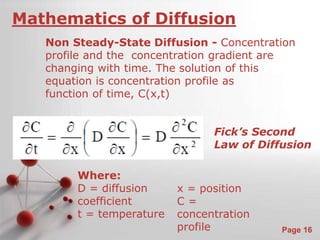
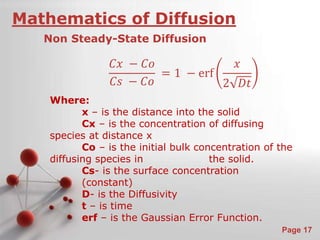
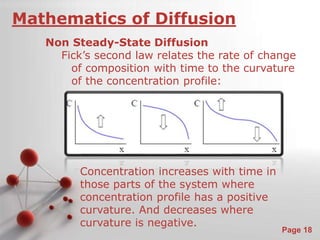
The document is a 23-page PowerPoint presentation about diffusion in materials science. It covers topics like the definition of diffusion, different types of diffusion like interdiffusion and self-diffusion, diffusion mechanisms like vacancy and interstitial diffusion, mathematics of diffusion using Fick's laws of steady-state and non-steady-state diffusion, examples calculations, and factors that affect diffusion rates such as temperature, microstructure, and the diffusing species. The presentation provides an overview of key concepts and equations regarding diffusion for a materials science class.