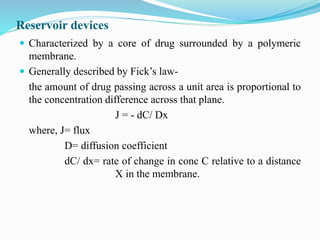
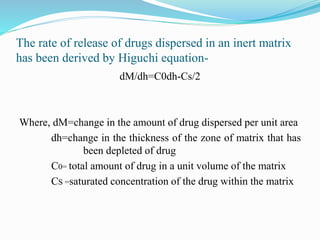
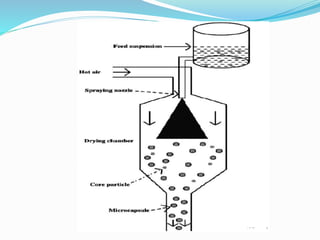
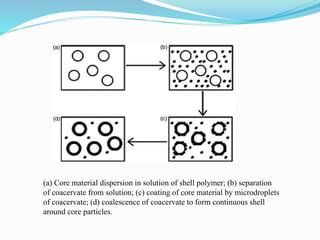
This document summarizes different types of diffusion controlled drug delivery systems. It describes reservoir and matrix devices. Reservoir devices consist of a drug core surrounded by a polymeric membrane, and drug release follows Fick's law of diffusion. Matrix devices involve drug dispersed throughout a polymer matrix, with drug on the surface dissolving first before diffusing out. The document provides the Higuchi equation that describes drug release from a matrix. It notes advantages like zero-order release for reservoir devices and lower risk of leakage for matrix devices, as well as disadvantages like need for removal after drug release. Methods for fabricating these devices like spray drying and coacervation are also summarized.