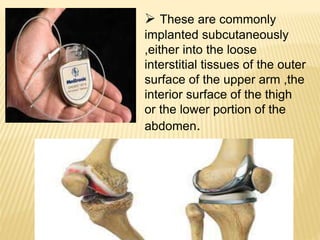
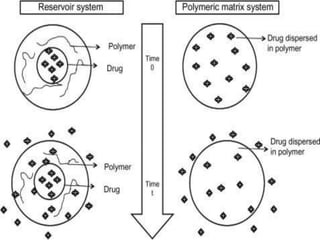
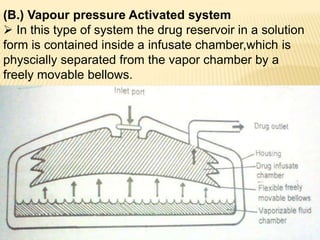
This document discusses implantable drug delivery systems. It defines implants as single-unit systems that deliver drugs at a controlled rate over a prolonged period. The document then covers the history, properties, advantages, disadvantages, mechanisms of drug release from implants, approaches to developing implants, evaluation criteria, and concludes that implants can provide controlled drug delivery but need to be biocompatible and avoid toxicity issues.