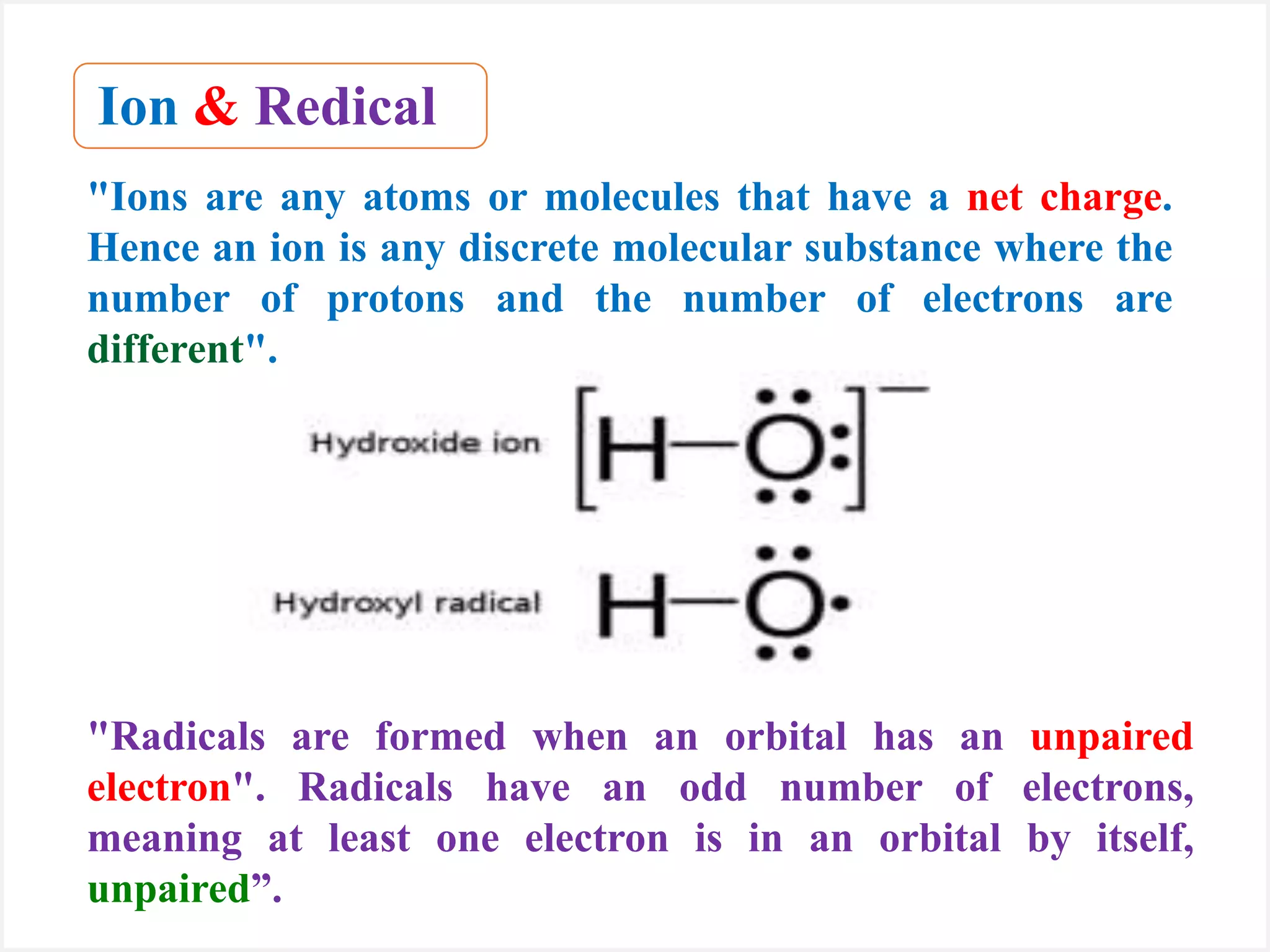
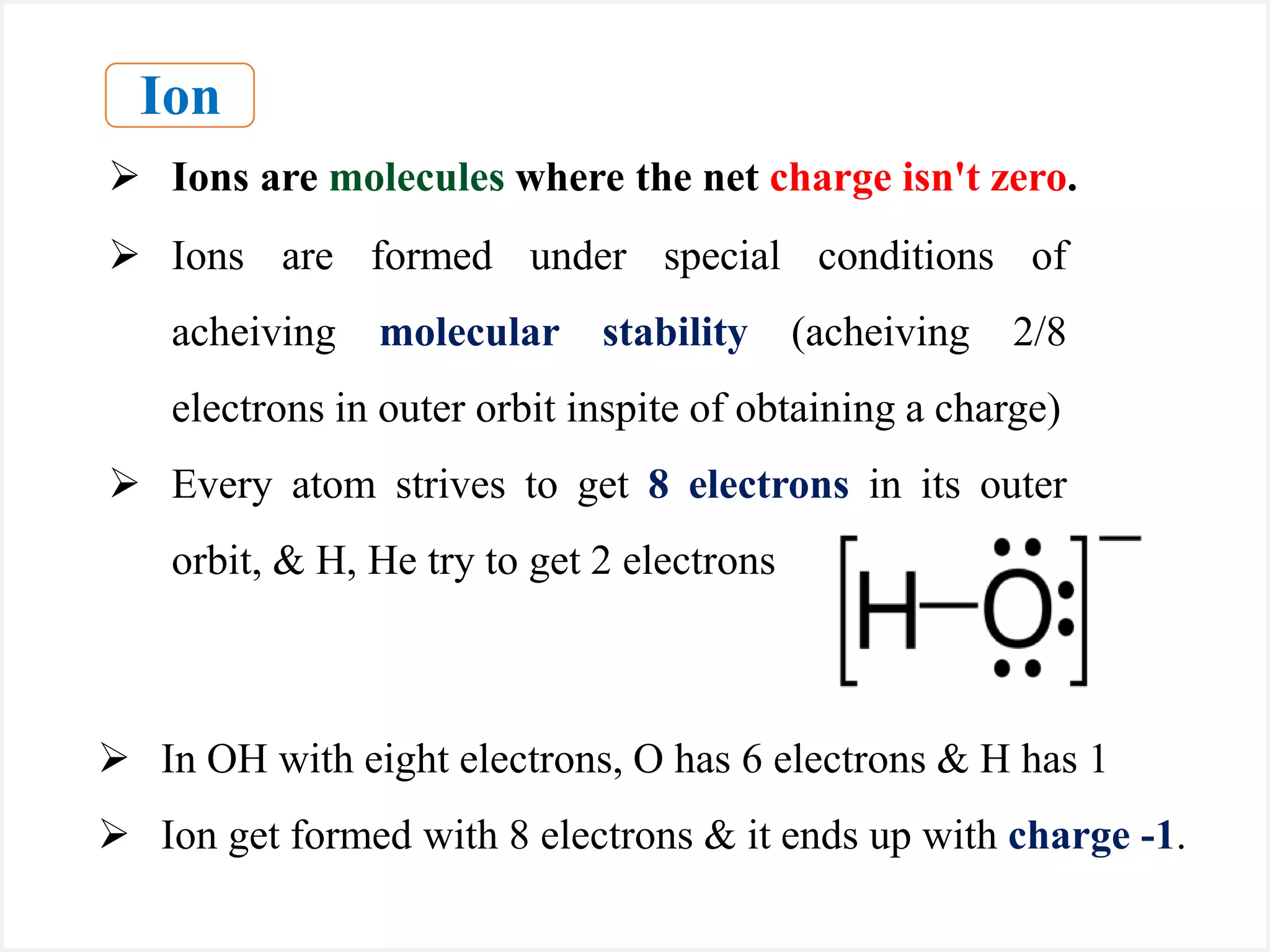
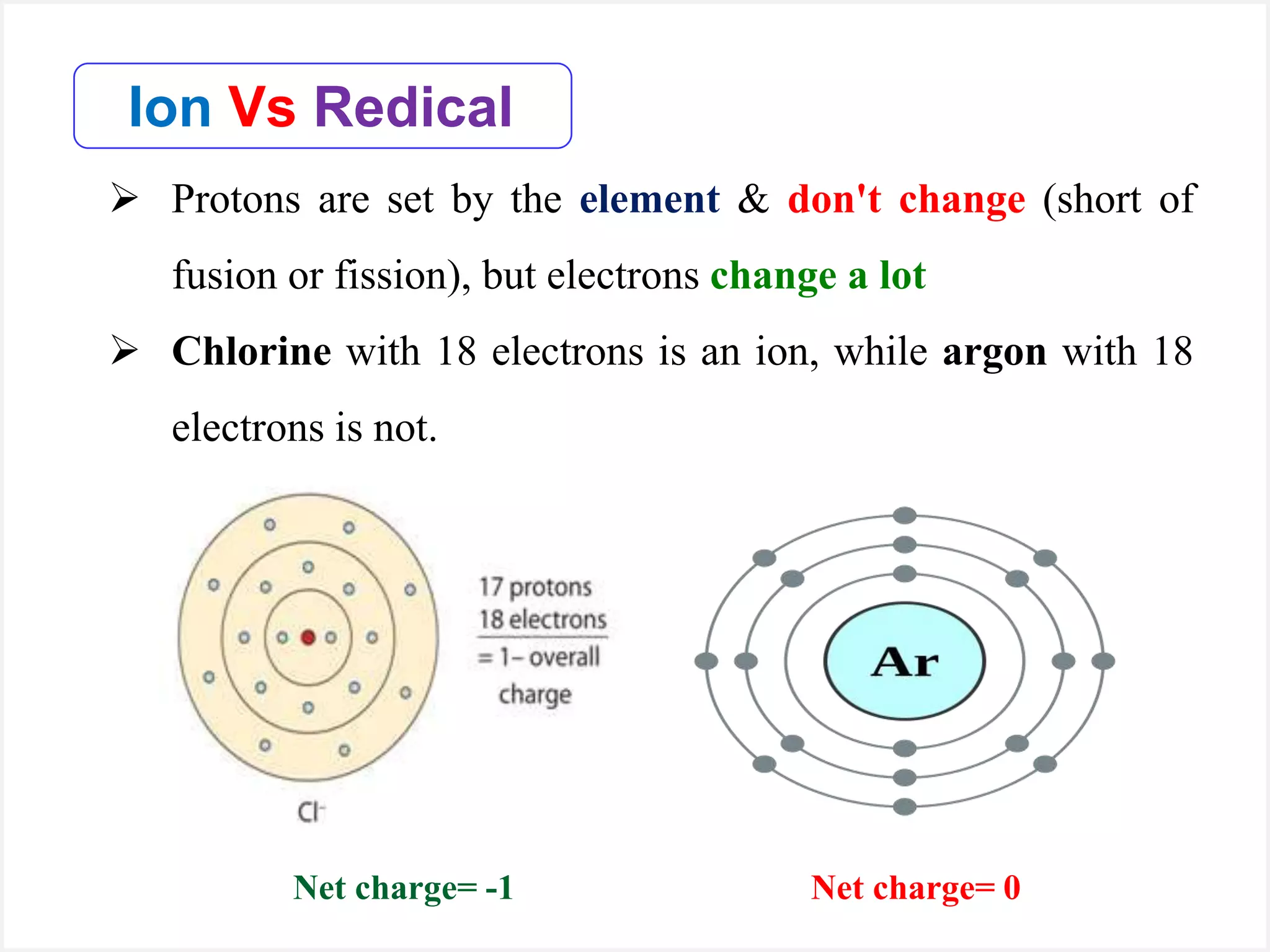
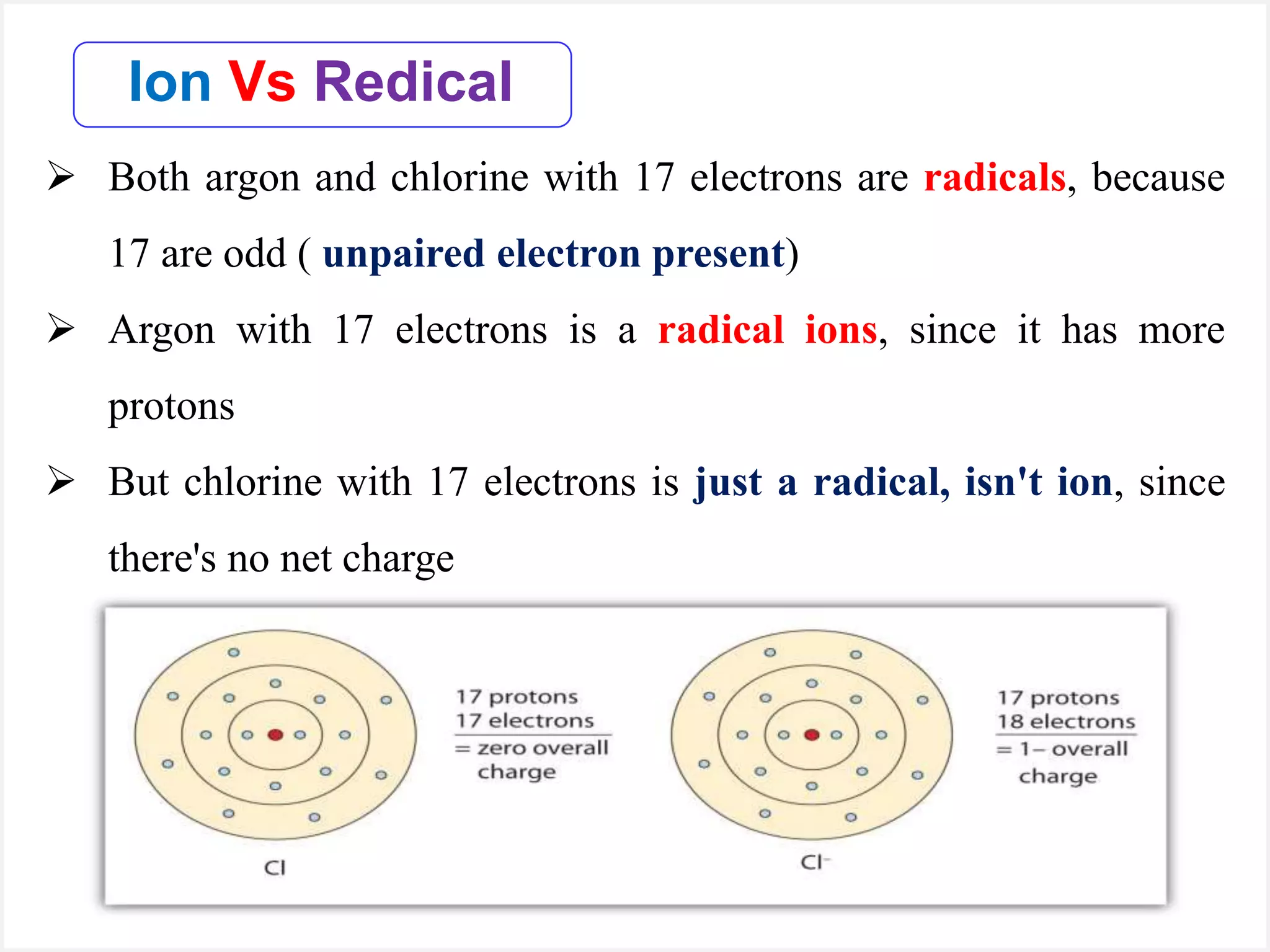
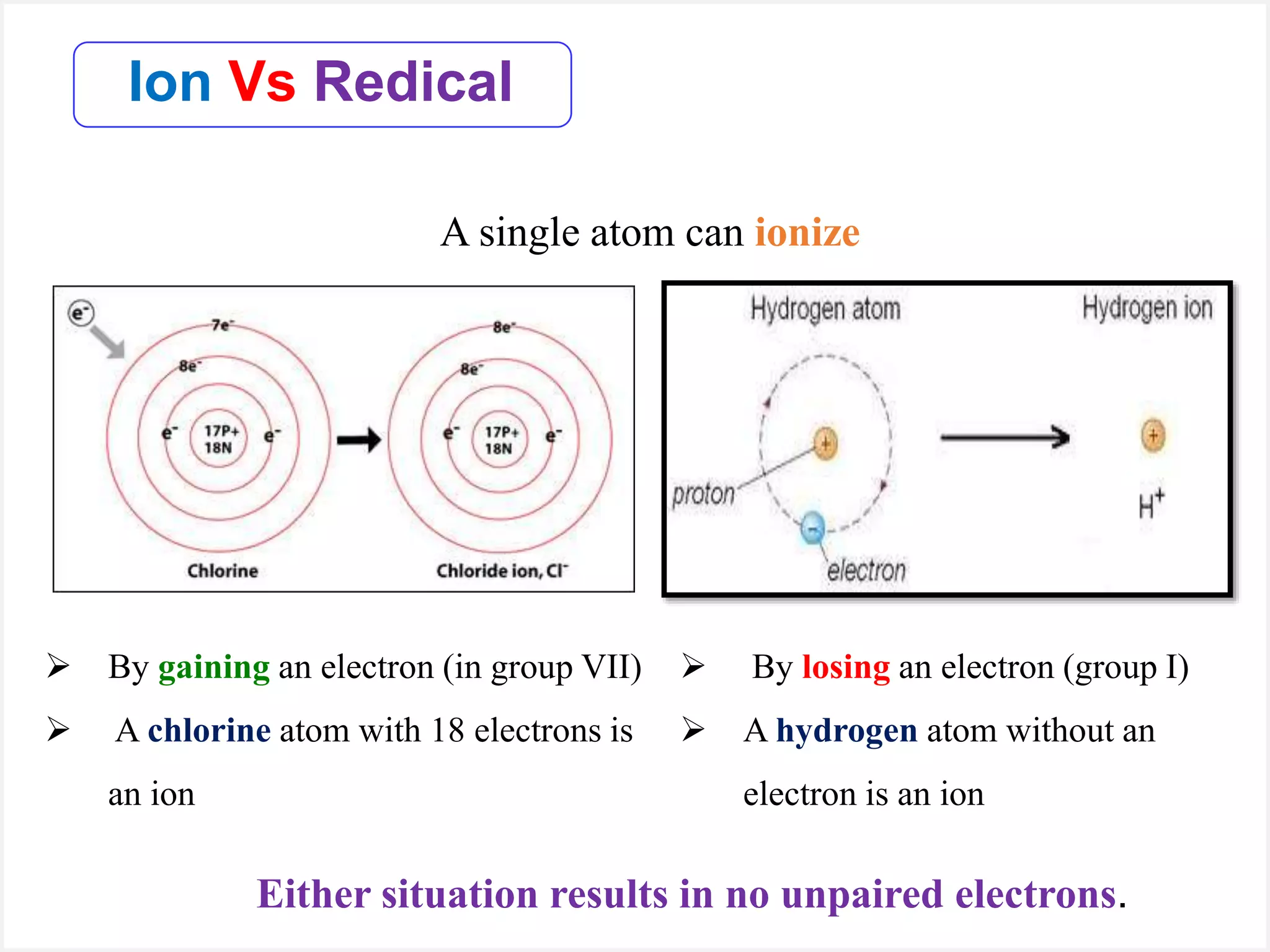
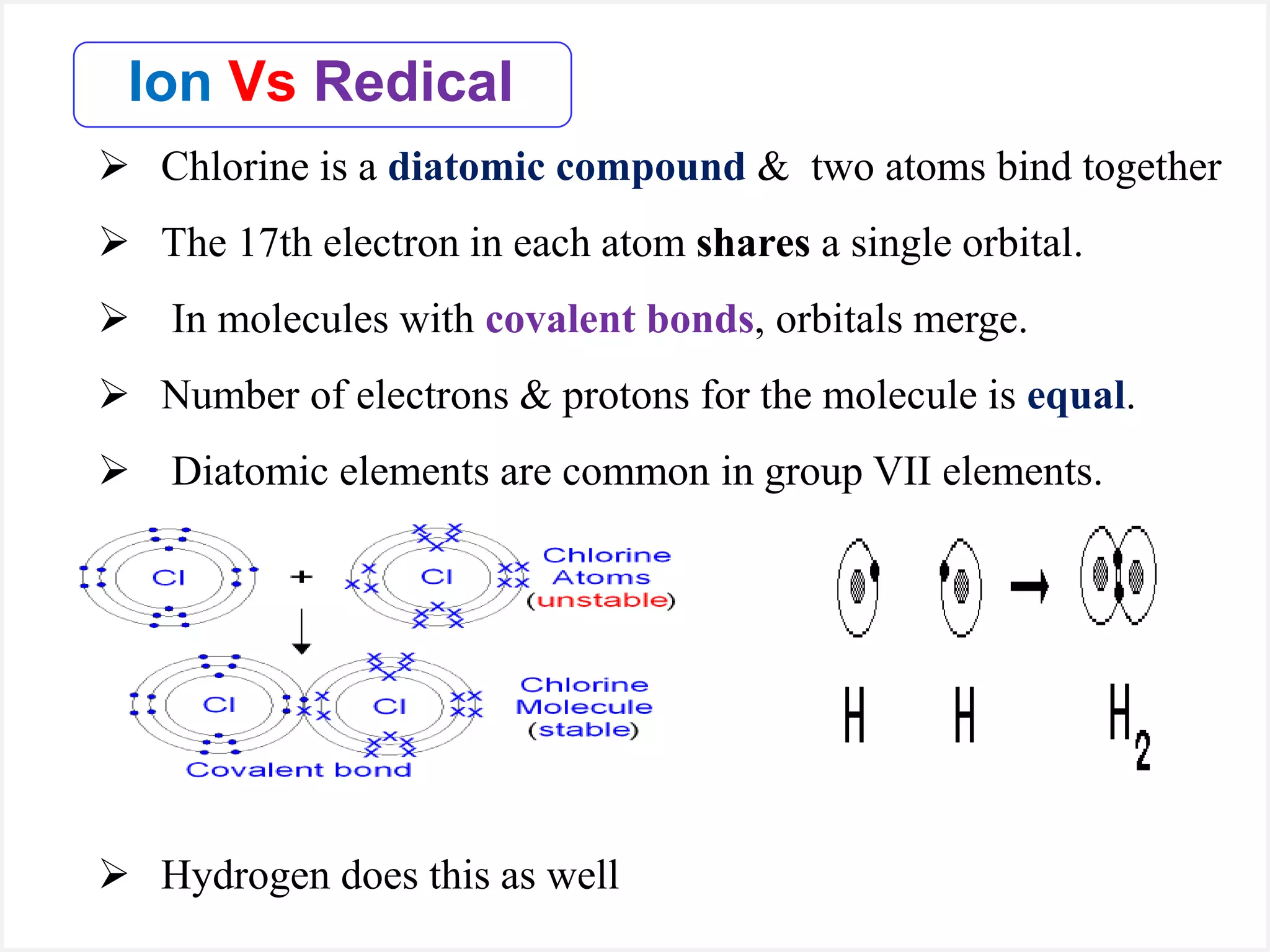
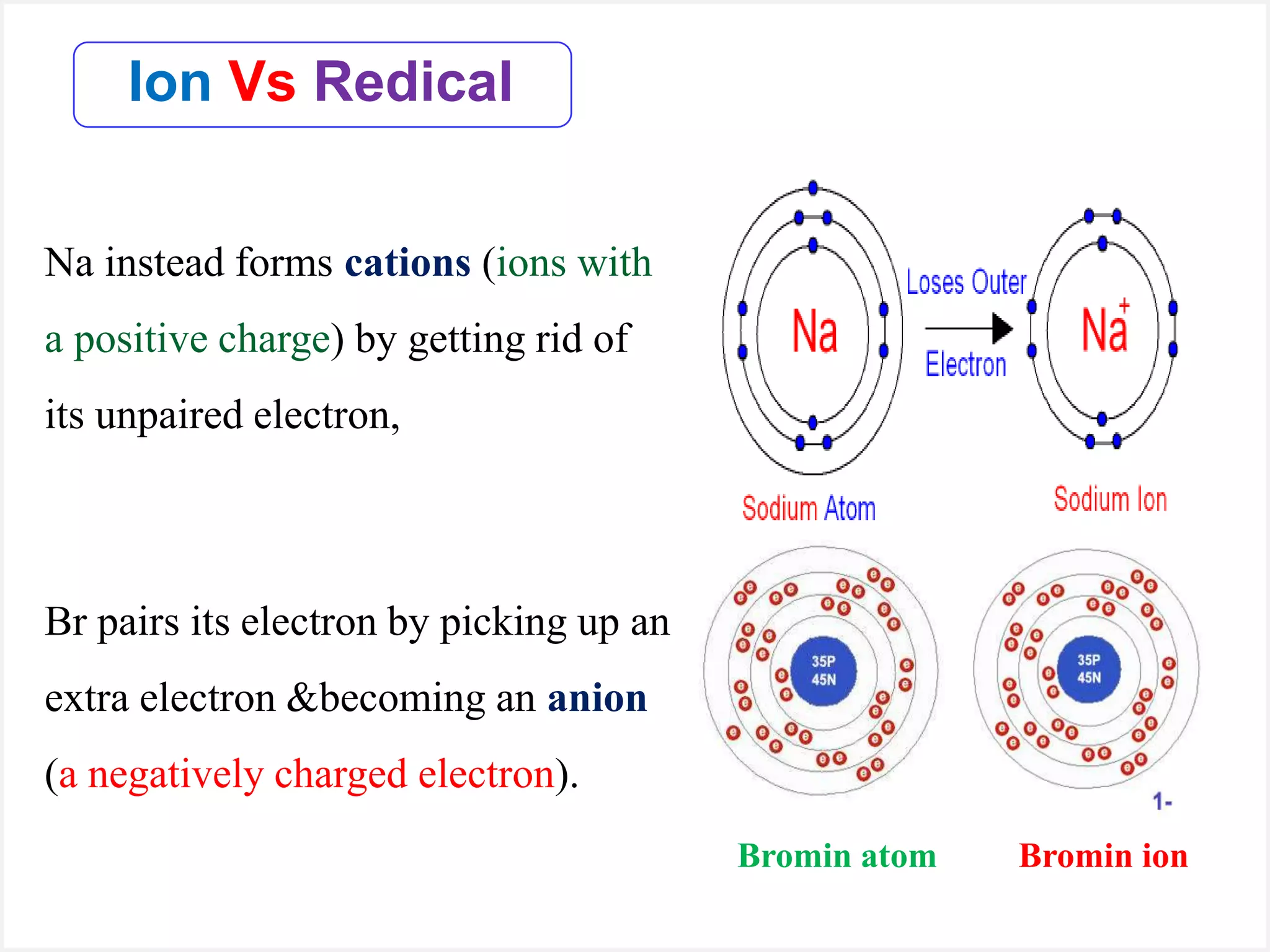
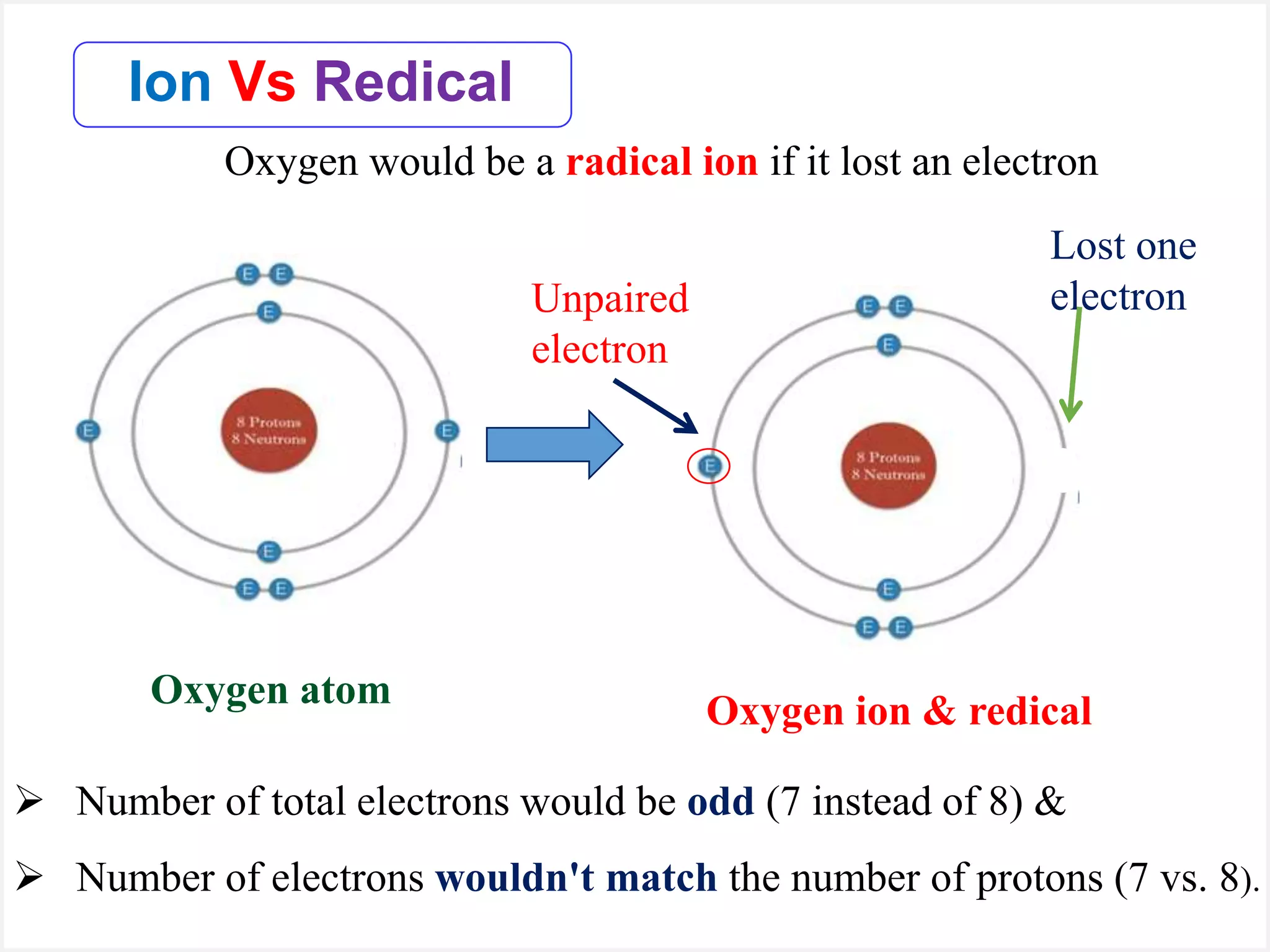
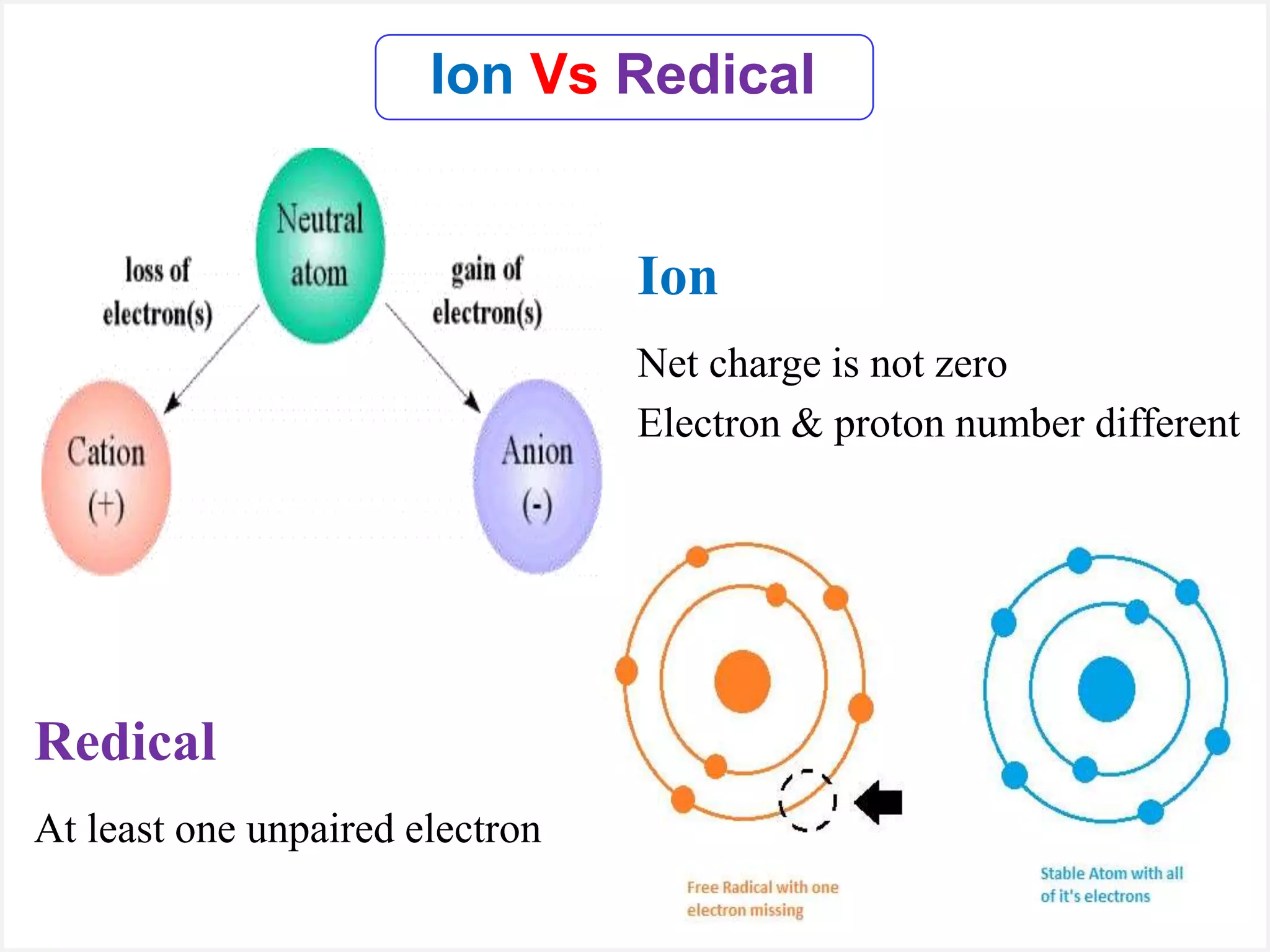
Ions are atoms or molecules with a net charge due to a difference in the number of protons and electrons, while radicals contain an unpaired electron and typically have no net charge. Ions are formed to achieve molecular stability, often resulting in a net charge, whereas radicals are defined by the presence of an unpaired electron, leading to odd electron counts. Examples include chlorine and argon, where chlorine can become an ion depending on electron gain or loss, while argon remains neutral.