Embed presentation
Downloaded 31 times





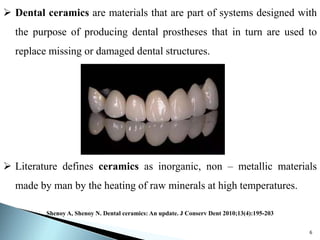











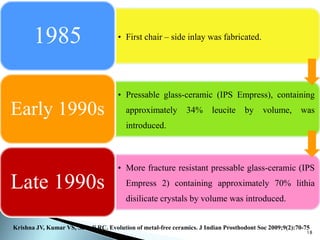




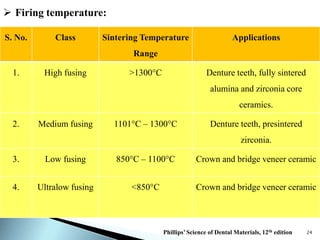

























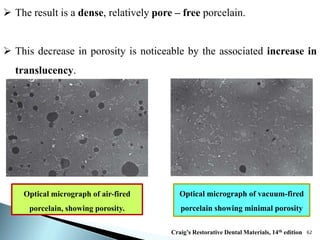


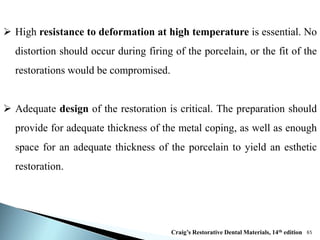




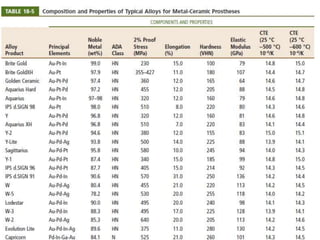
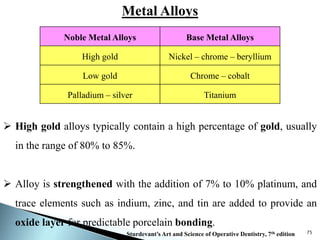







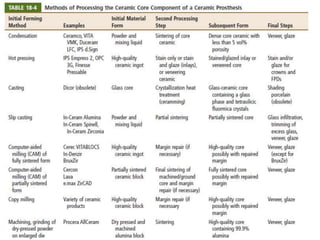


















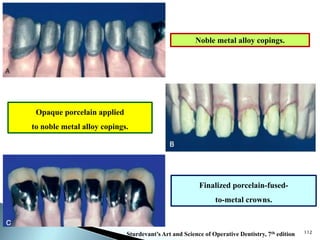











































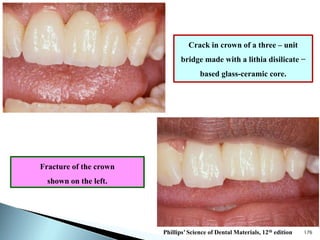











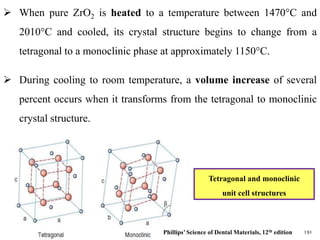

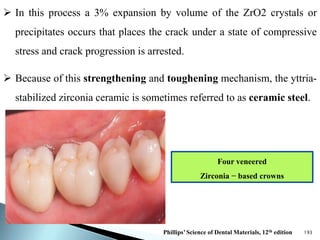































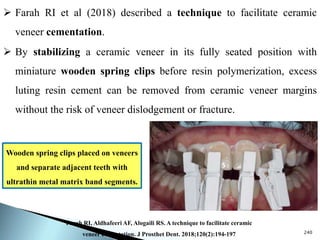



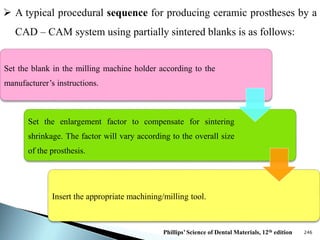
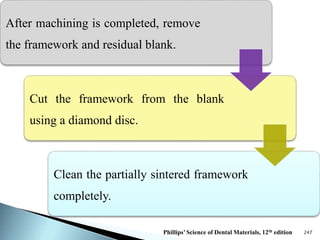
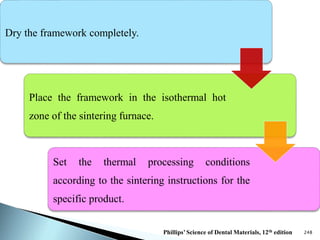
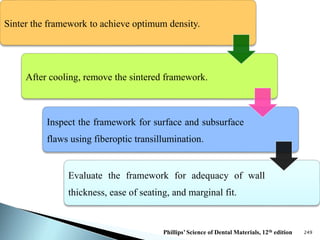




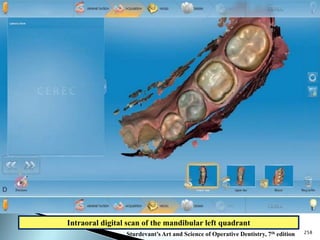
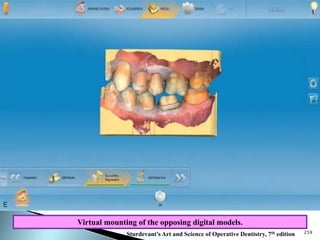
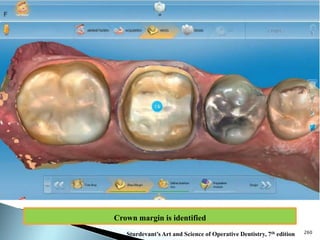
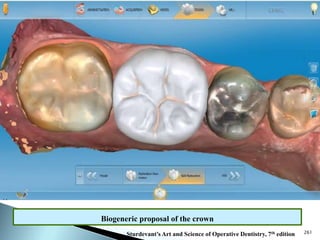
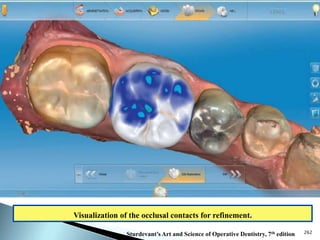
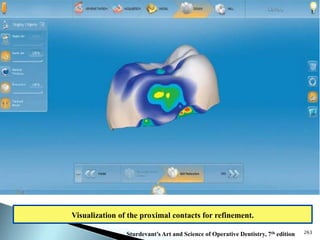
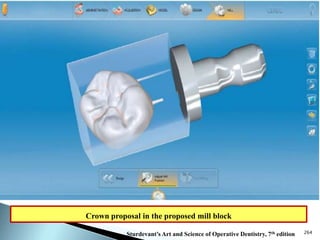
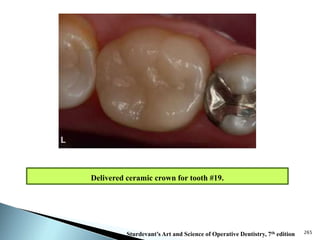























Dental ceramics are ideal restorative materials due to their biocompatibility, durability, and aesthetic qualities, making them suitable for replacing damaged dental structures. These inorganic, non-metallic materials are produced through high-temperature heating of raw minerals and can mimic the appearance of natural teeth. Key properties include excellent resistance to compressive stresses, although they are more susceptible to tensile and shear stresses, presenting a brittle nature.

































































































































































































































































































