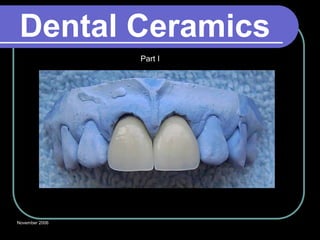
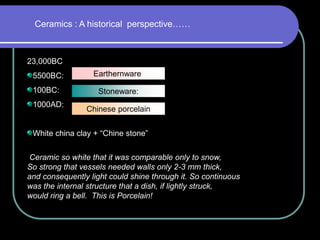
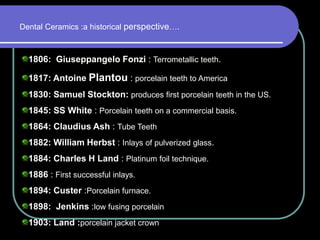
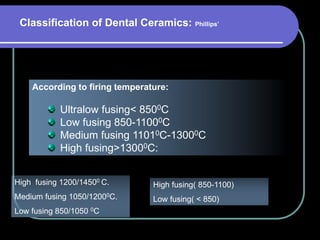
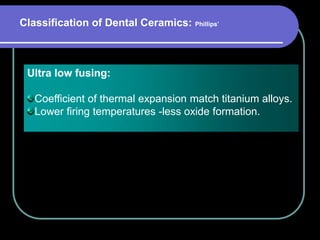
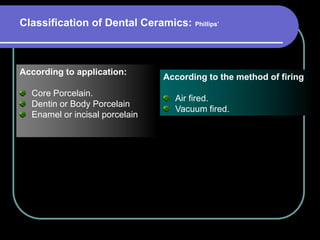
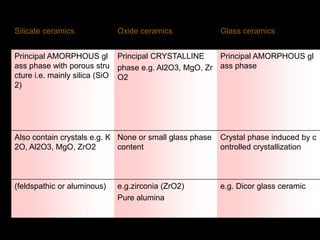
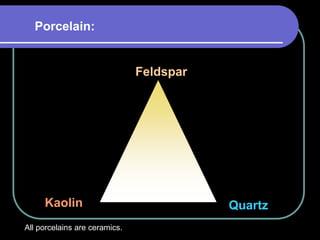
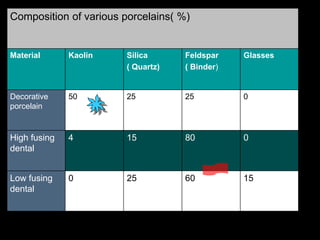
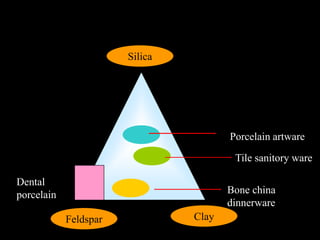
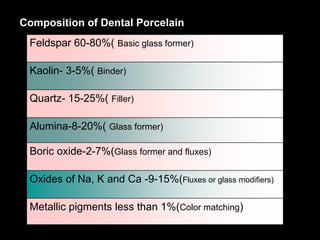
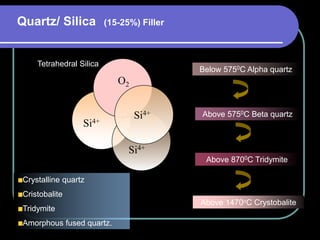
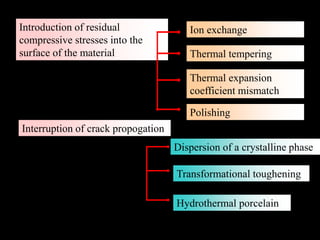
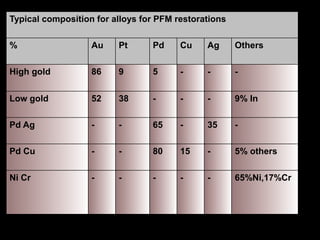
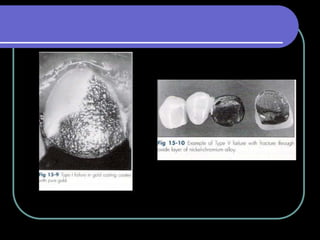
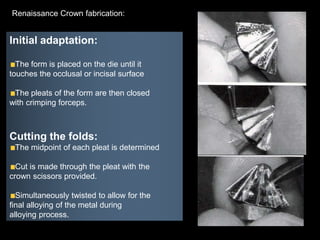
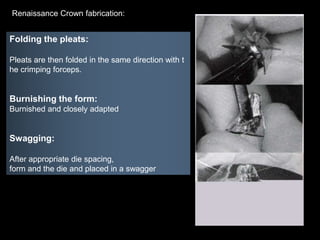
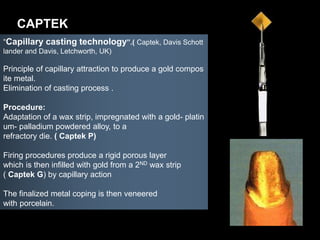
Dental ceramics have been used in dentistry for hundreds of years, with early attempts to imitate Chinese porcelain in the 1700s. Modern dental ceramics are classified based on their composition, firing temperature, microstructure, and intended use. They provide esthetic and durable alternatives to metallic restorations due to properties like biocompatibility, color stability, and strength. Common types include feldspathic porcelain, lithium disilicate glass ceramic, and zirconia.