This document provides an overview of dental amalgam. It begins with a brief introduction, then discusses the history of amalgam use dating back to ancient China. The document outlines various classifications of amalgam and lists indications and contraindications for its use. Advantages include ease of use and strength, while disadvantages include esthetics and weakness of tooth structure. The document discusses the composition of amalgam, including the roles of individual components like silver, tin and copper. It also summarizes the amalgamation reaction and properties of amalgam like strength, creep and corrosion resistance.










































![RECENT ADVANCES
RESIN COATED AMALGAM:
• Mertz-fairhurst and others evaluated bonded and sealed composite restorations
placed directly over frank cavitated lesions extending into dentin versus sealed
conservative amalgam restorations and conventional unsealed amalgam
restorations.
• The results indicate that both types of sealed restorations exhibited superior
clinical performance and longevity compared with unsealed amalgam restorations
over a period of 10 years.
Mertz-Fairhurst EJ, Curtis JW, Jr, Ergle JW, Rueggeberg RA, Adair SM. Ultraconservative and cariostatic sealed restorations: Results at year
10. J Am Dent Assoc. 1998;129:55–66. [PubMed] [Google Scholar]](https://image.slidesharecdn.com/amalgam-200417081631/85/Amalgam-44-320.jpg)








![• Vrijhoef MM, Letzel H: Creep versus marginal fracture of amalgam restorations, J Oral Rehabil
13:299-303, 1986.
• Mertz-Fairhurst EJ, Curtis JW, Jr, Ergle JW, Rueggeberg RA, Adair SM. Ultraconservative and
cariostatic sealed restorations: Results at year 10. J Am Dent Assoc. 1998;129:55–
66. [PubMed] [Google Scholar]
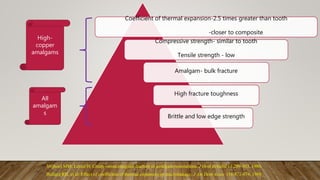
• Bullard RH, et al: Effect of coefficient of thermal expansion on microleakage, J Am Dent Assoc
116:871-874, 1988.
• Eames WB: Preparation and condensation of amalgam with a low mercury/alloy ratio. J Am Dent
Assoc 58:78–83, 1959.
• Lloyd CH, Adamson M: The fracture toughness of amalgam. J Oral Rehab 12:59–68, 1985.
• Clarkson TW. The toxicology of mercury. Critical reviews in clinical laboratory sciences. 1997 Jan
1;34(4):369-403.
• Skare I, Bergström T, Engqvist A, Weiner JA. Mercury exposure of different origins among
dentists and dental nurses. Scandinavian journal of work, environment & health. 1990 Oct](https://image.slidesharecdn.com/amalgam-200417081631/85/Amalgam-53-320.jpg)
