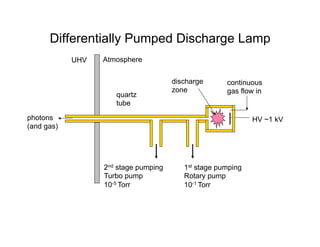
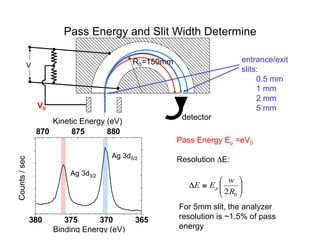
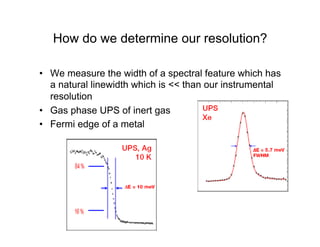
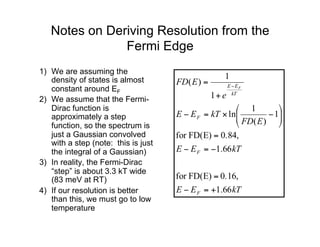
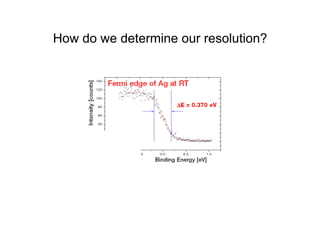
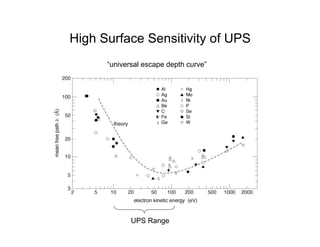
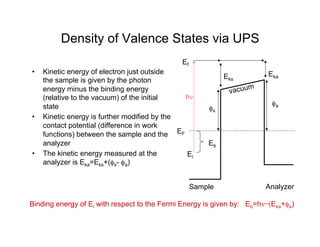
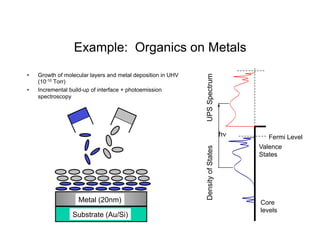
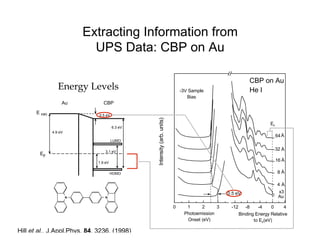
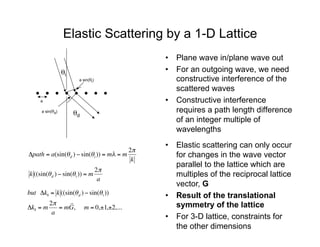
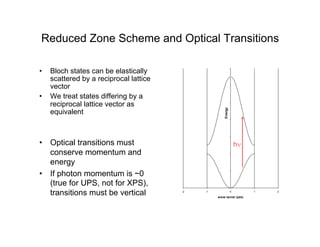
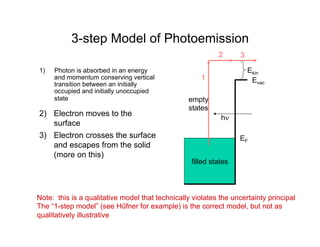
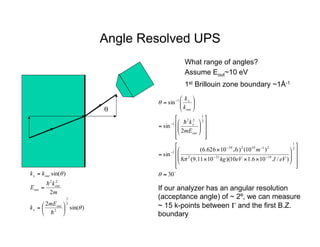
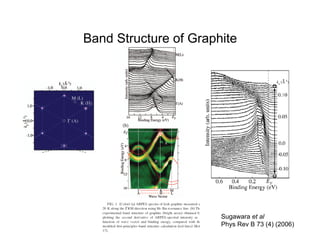
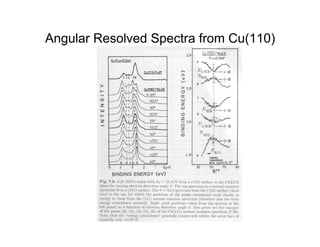
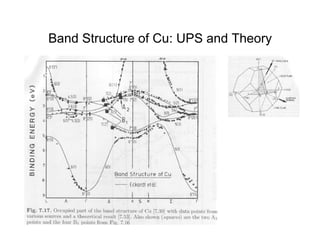
Ultraviolet photoelectron spectroscopy (UPS) probes valence states with higher energy resolution than XPS due to using higher photon energies in the vacuum ultraviolet range. Two common methods for producing VUV photons are synchrotron radiation, which provides high photon flux but requires expensive facilities, and differentially pumped gas discharge lamps, which can be housed in a university lab but have limited tunability. UPS provides high surface sensitivity due to the short escape depth of photoelectrons. Angle-resolved UPS allows measurement of crystal band structure by varying the emission angle to determine momentum components parallel to the surface.