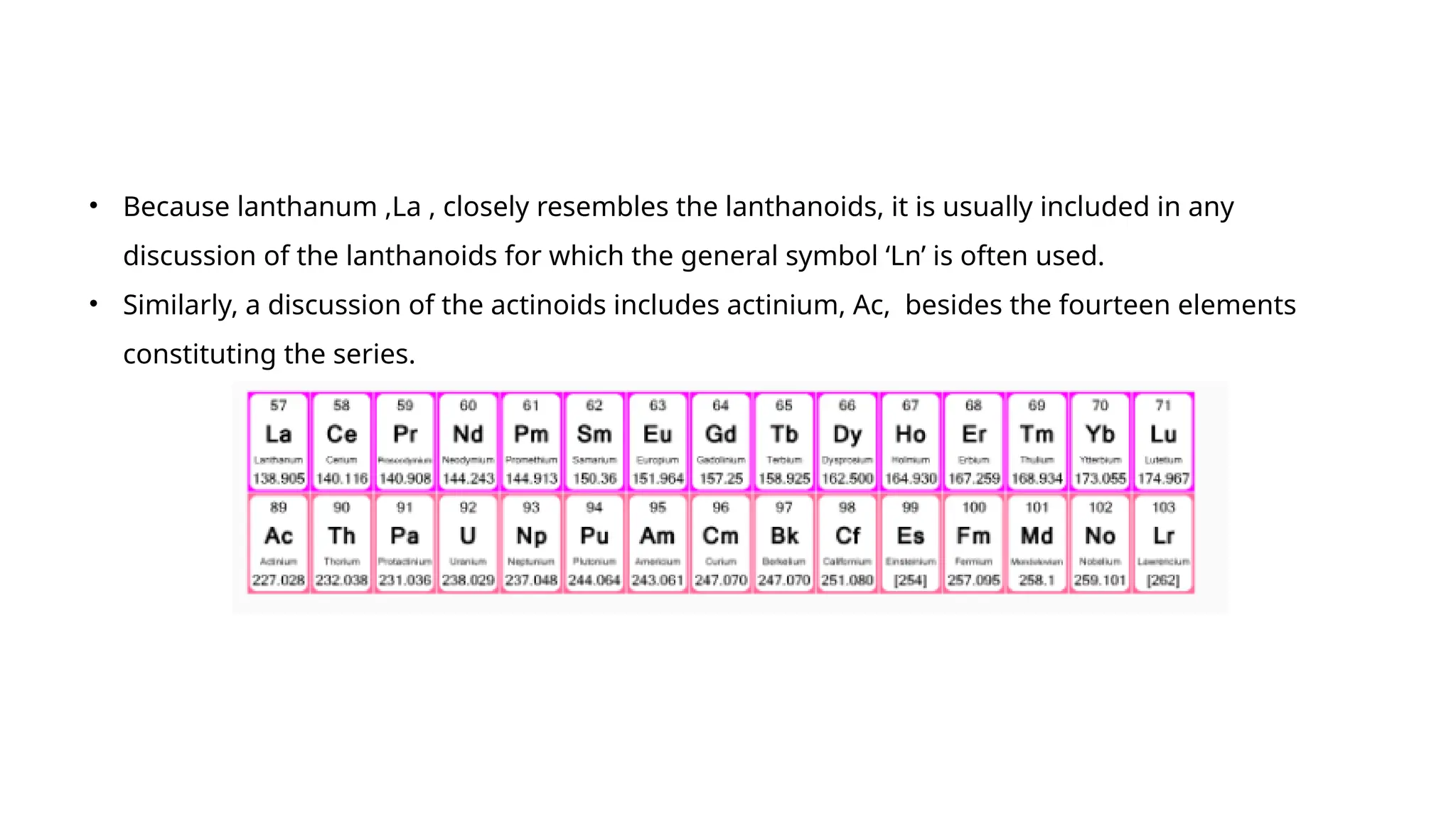
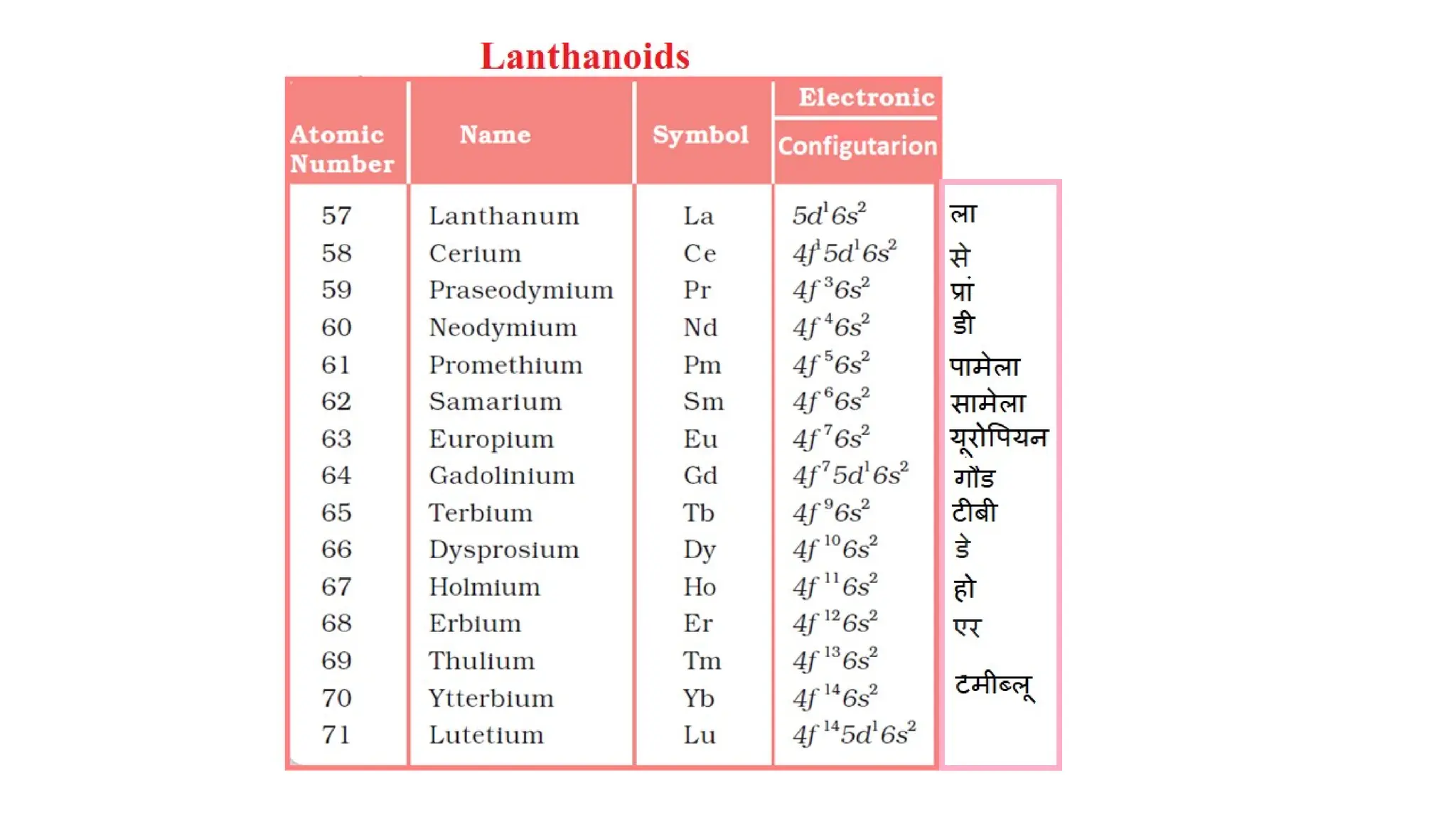
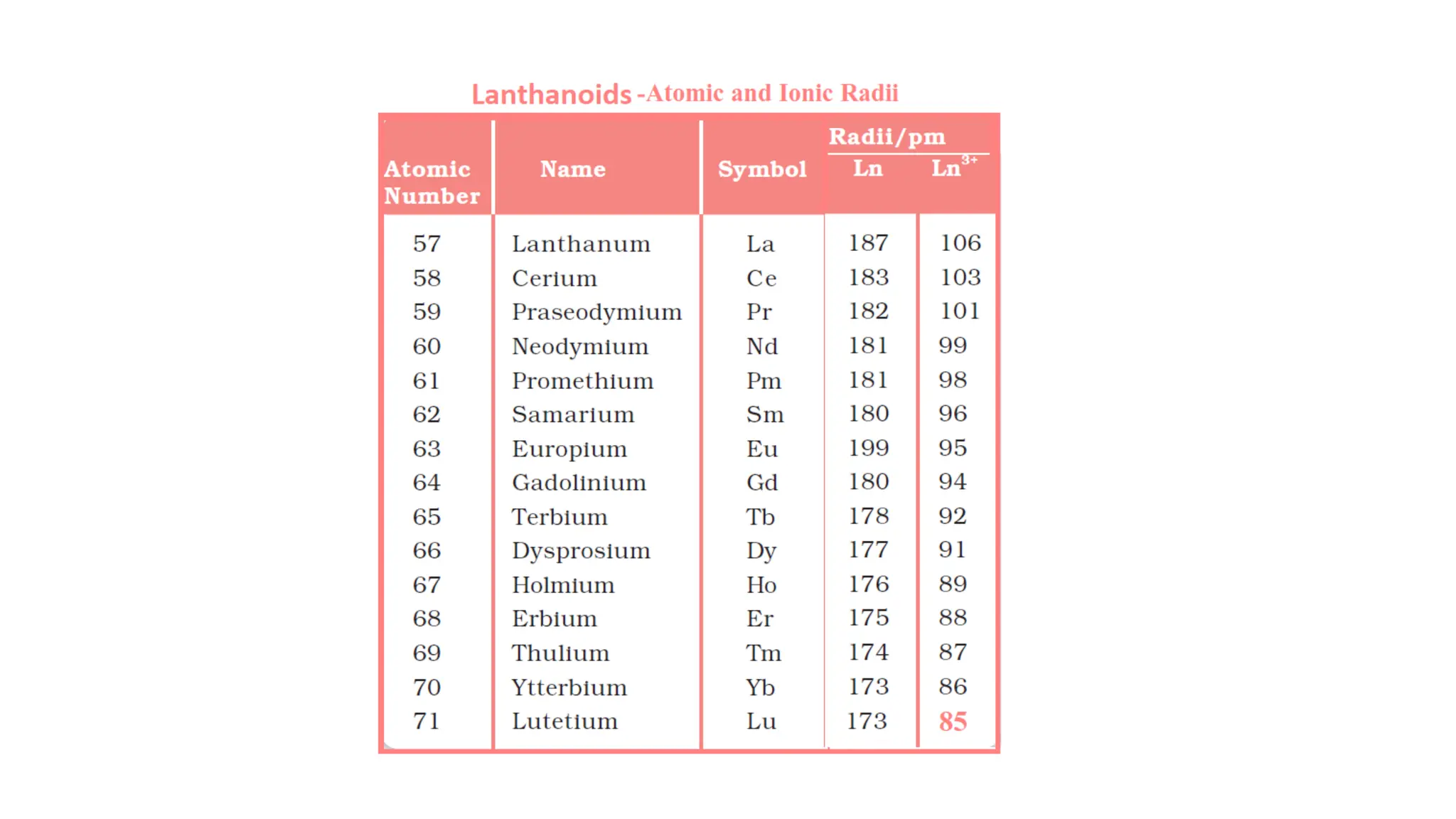
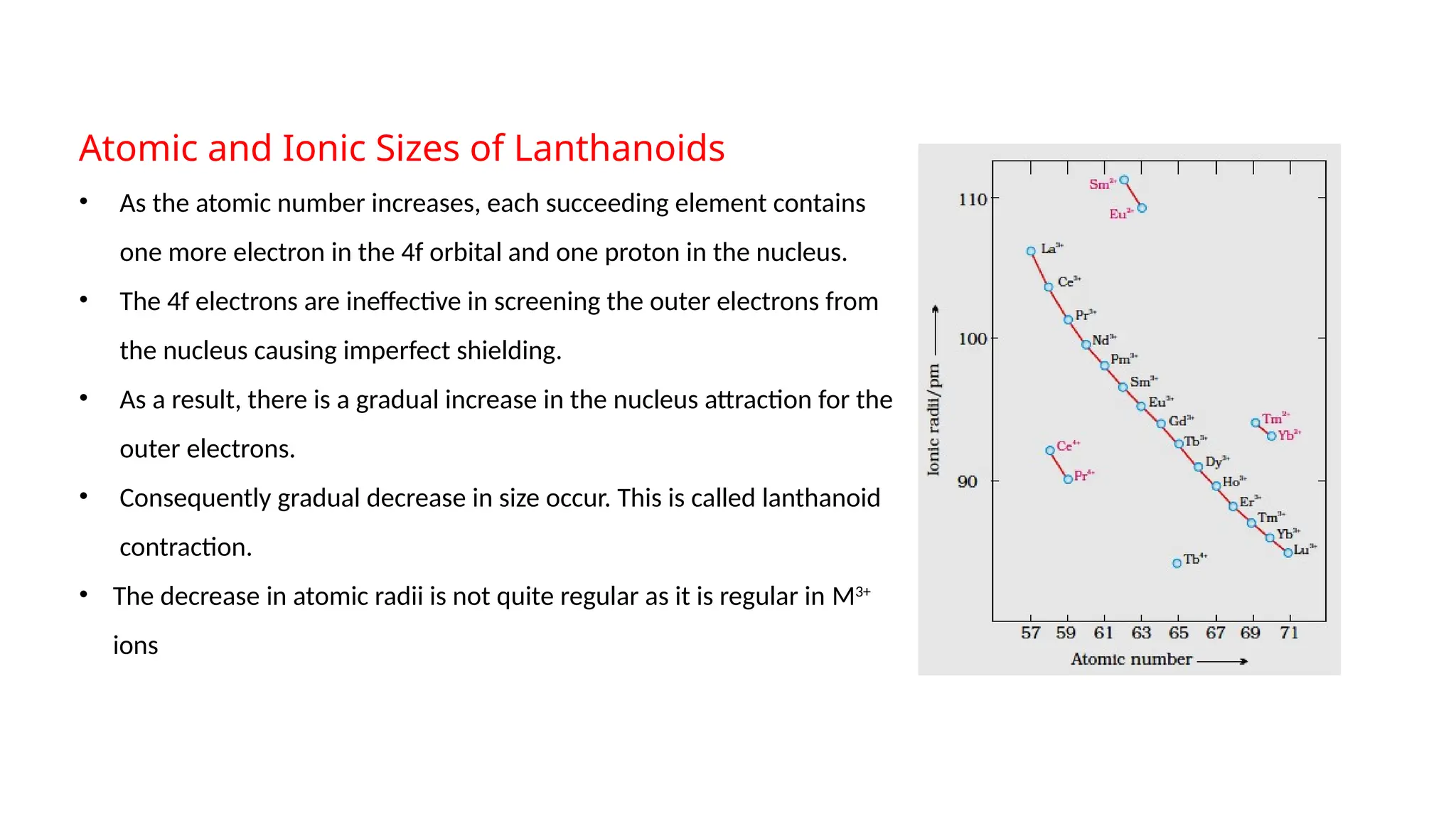
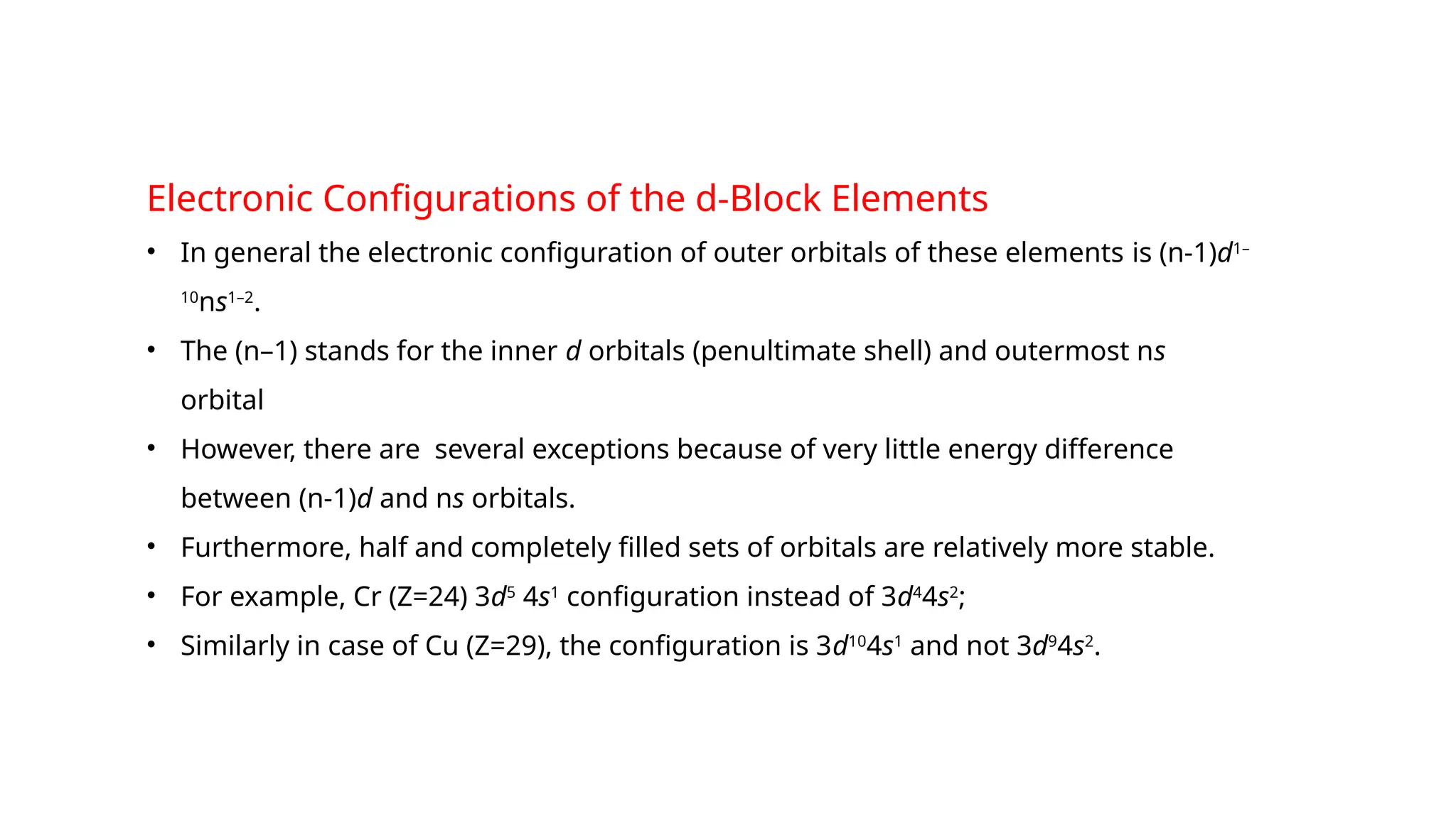
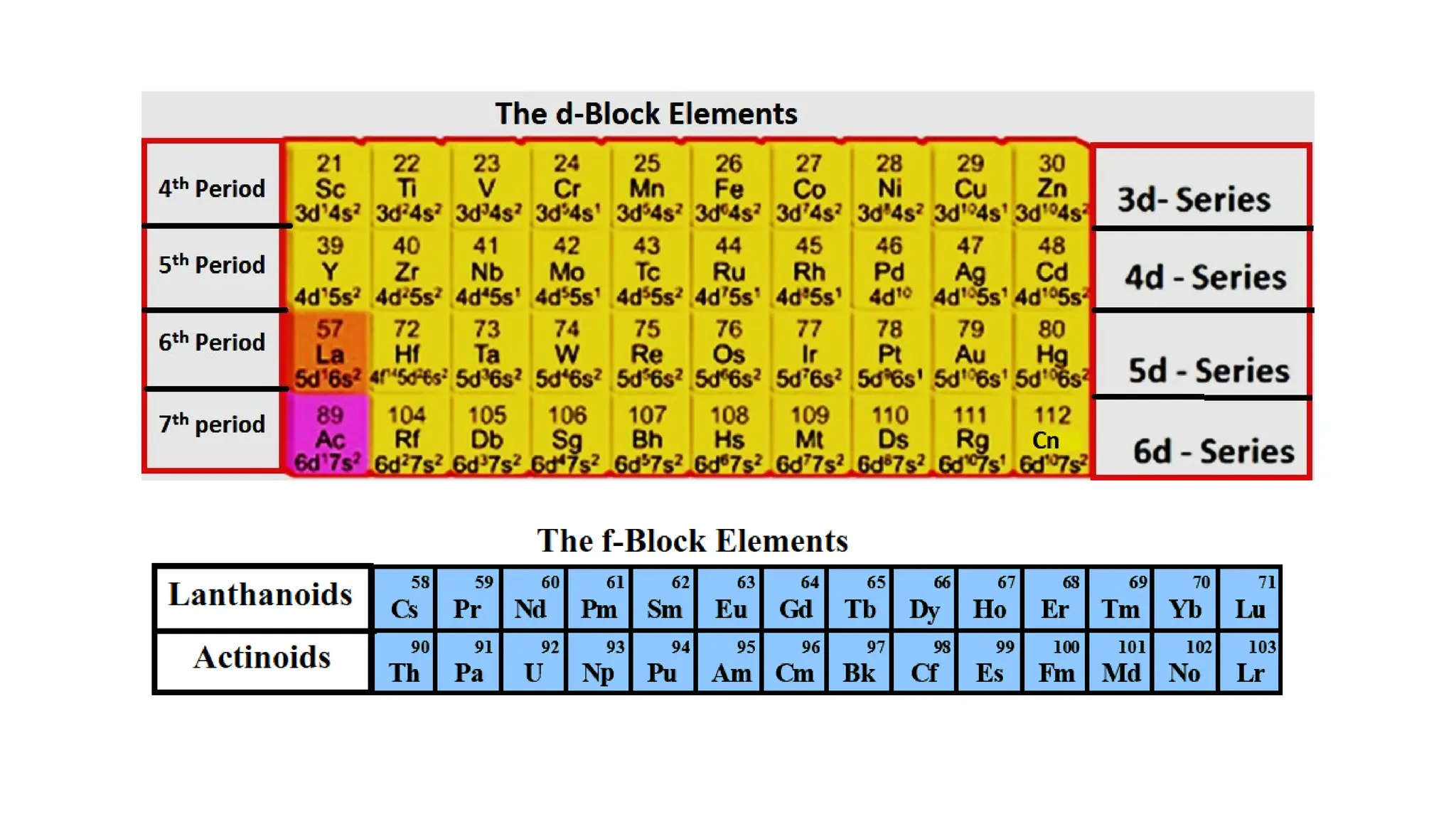
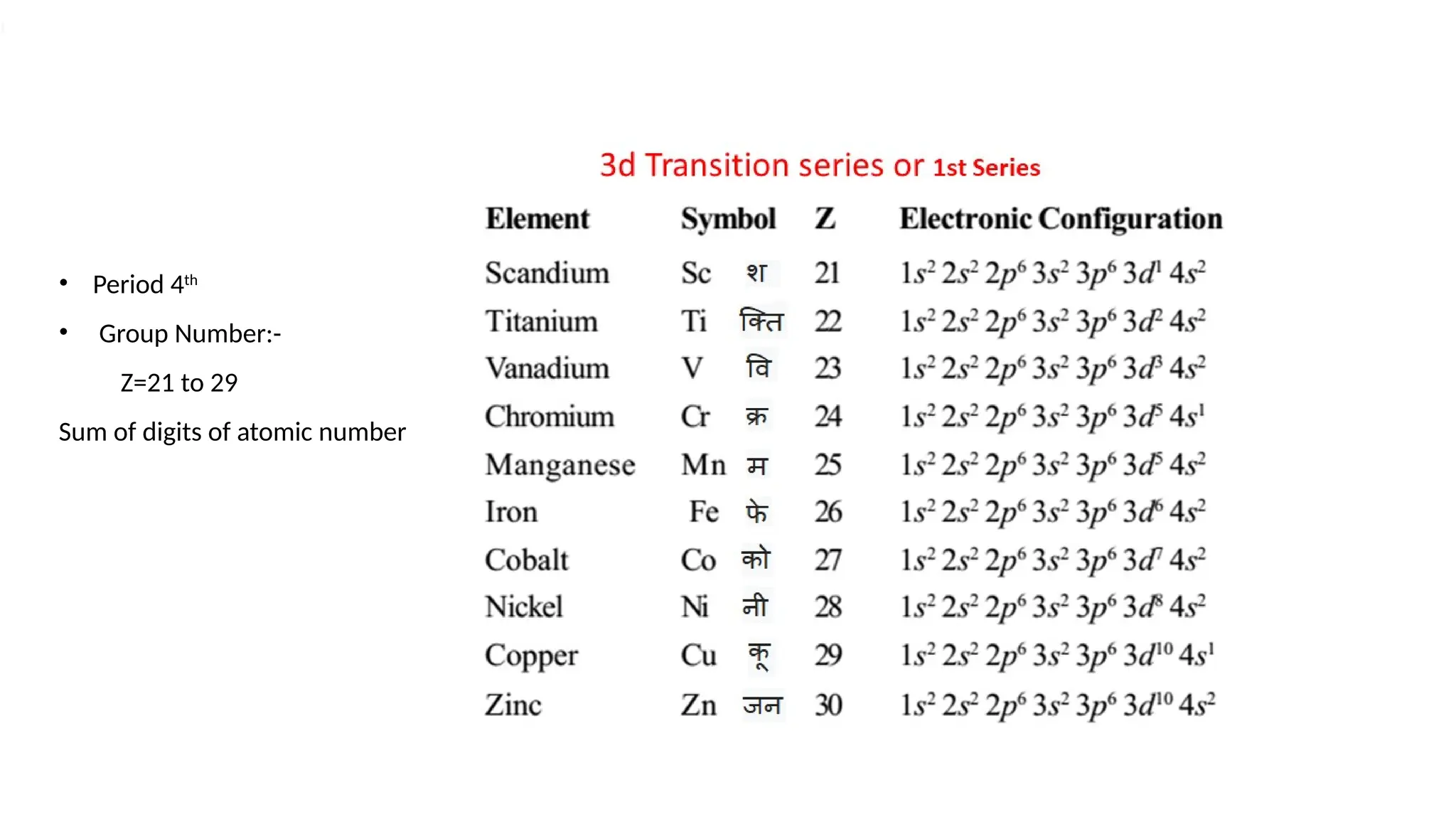
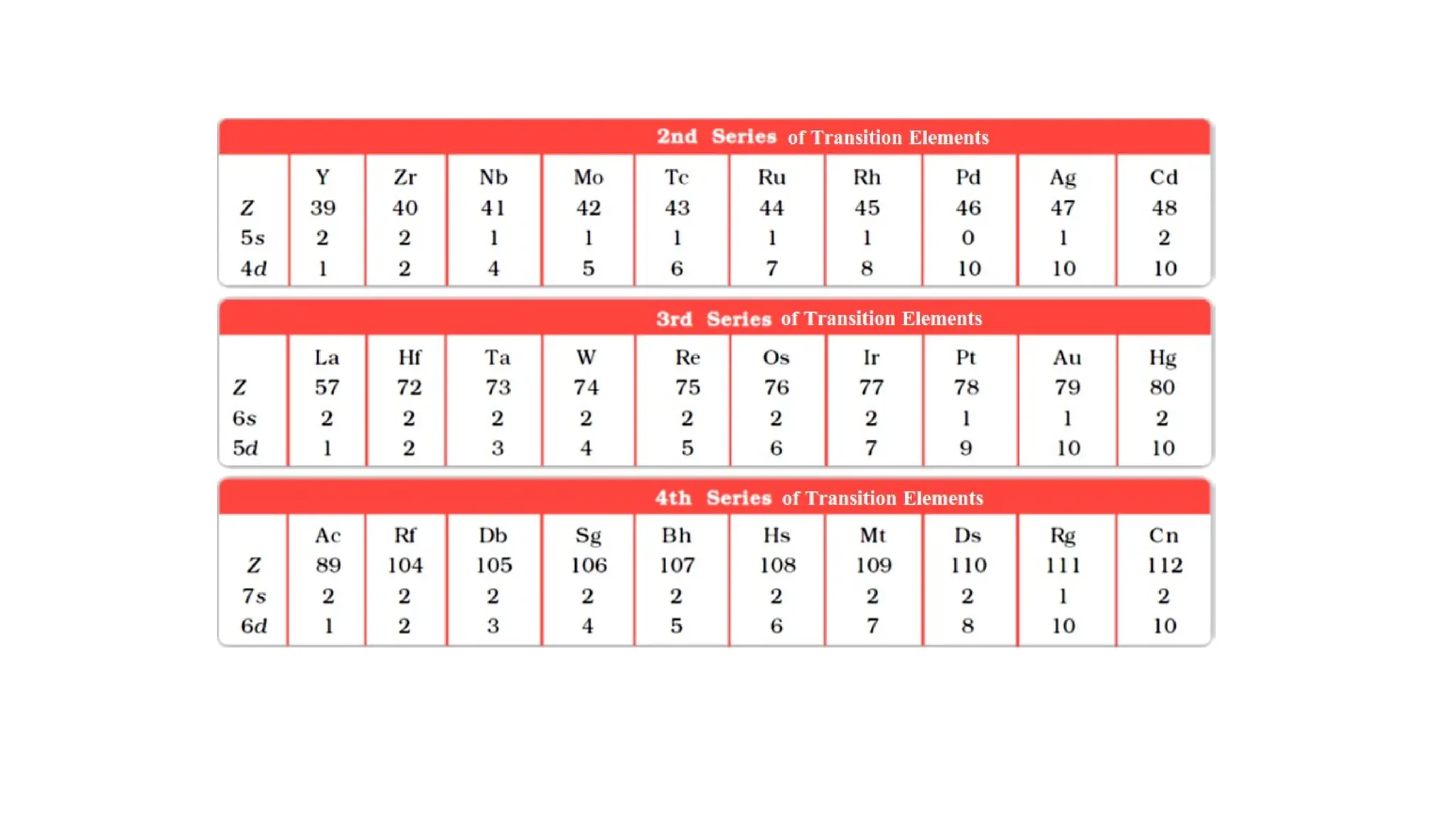
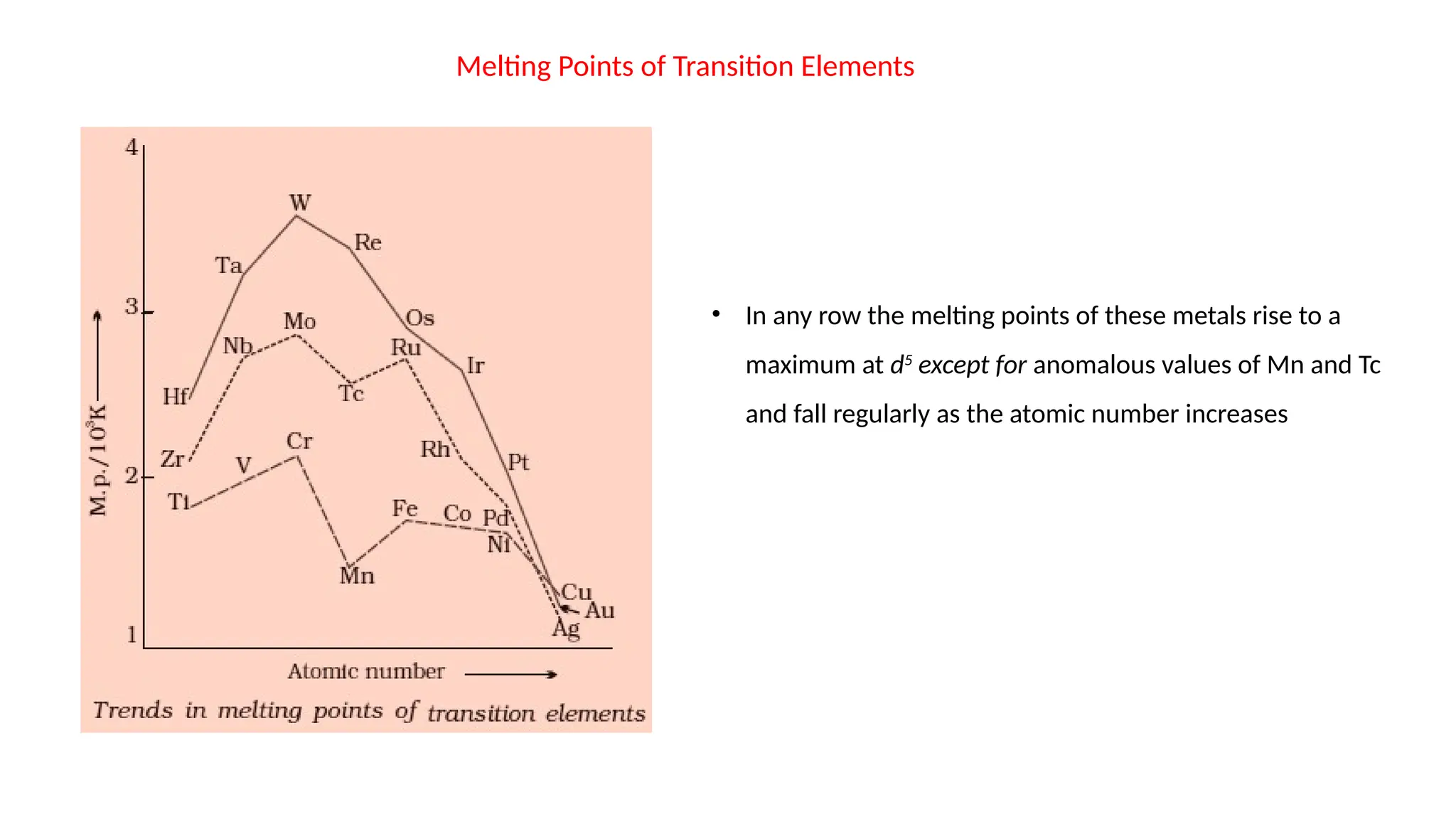
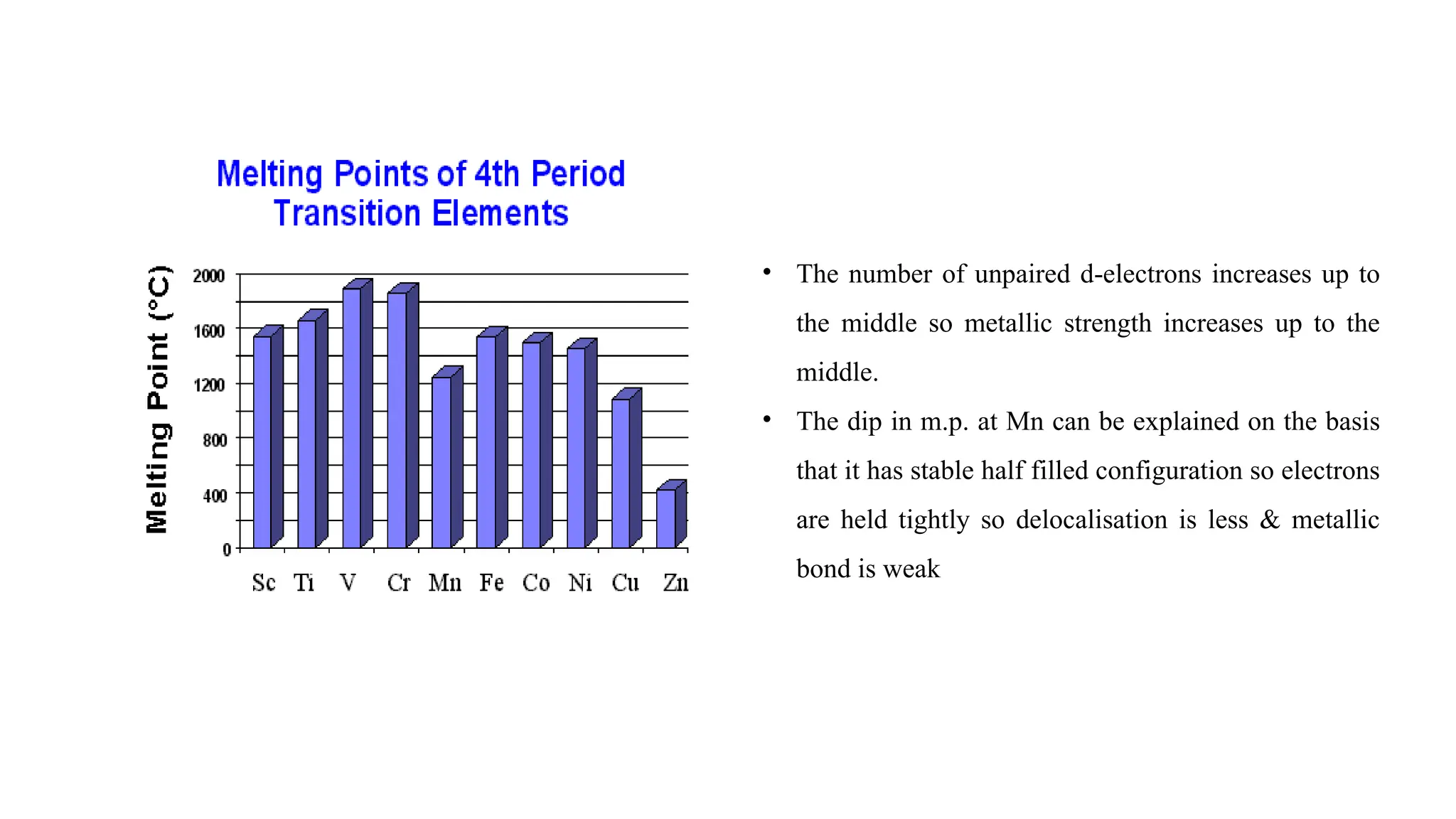
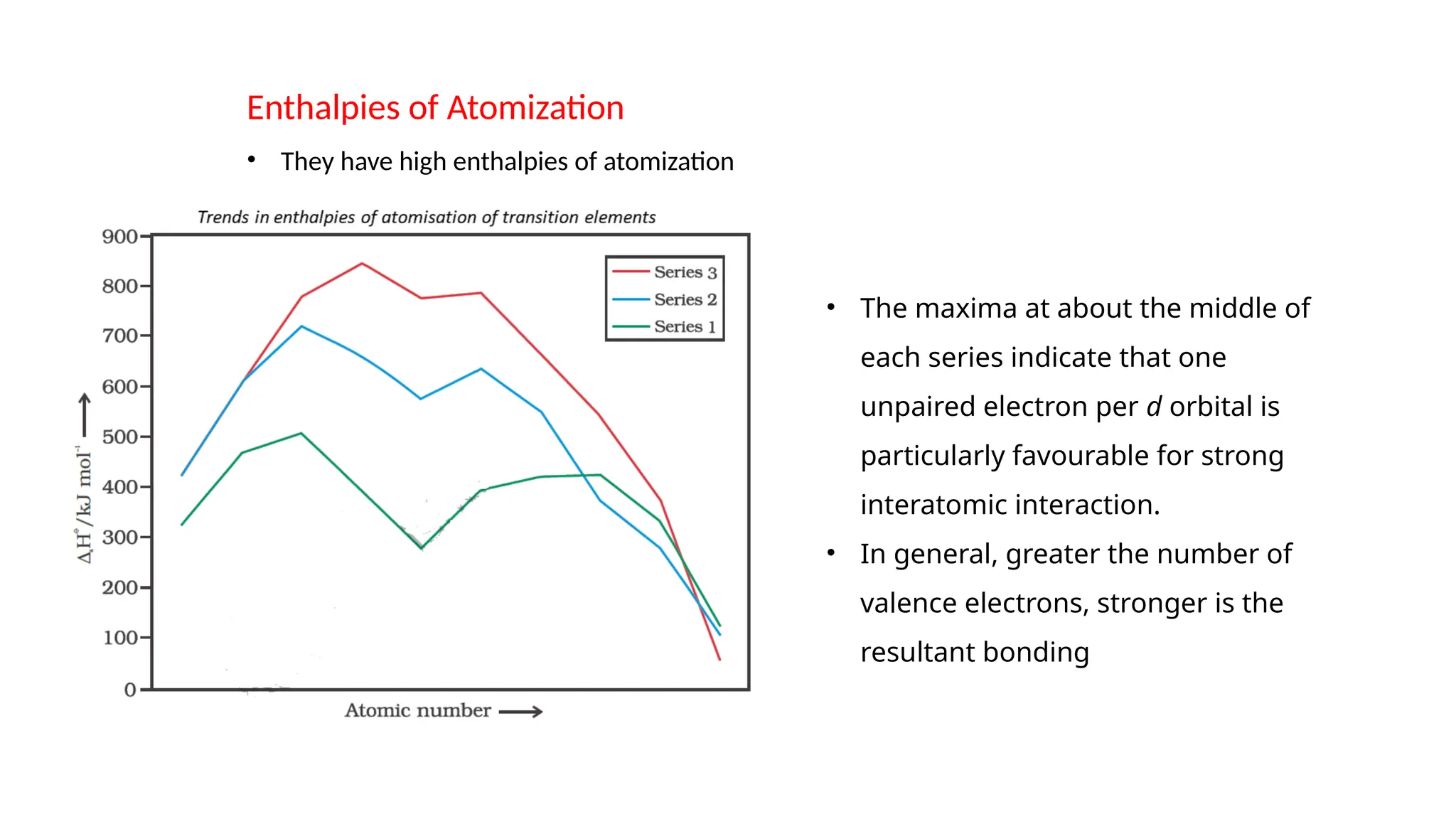
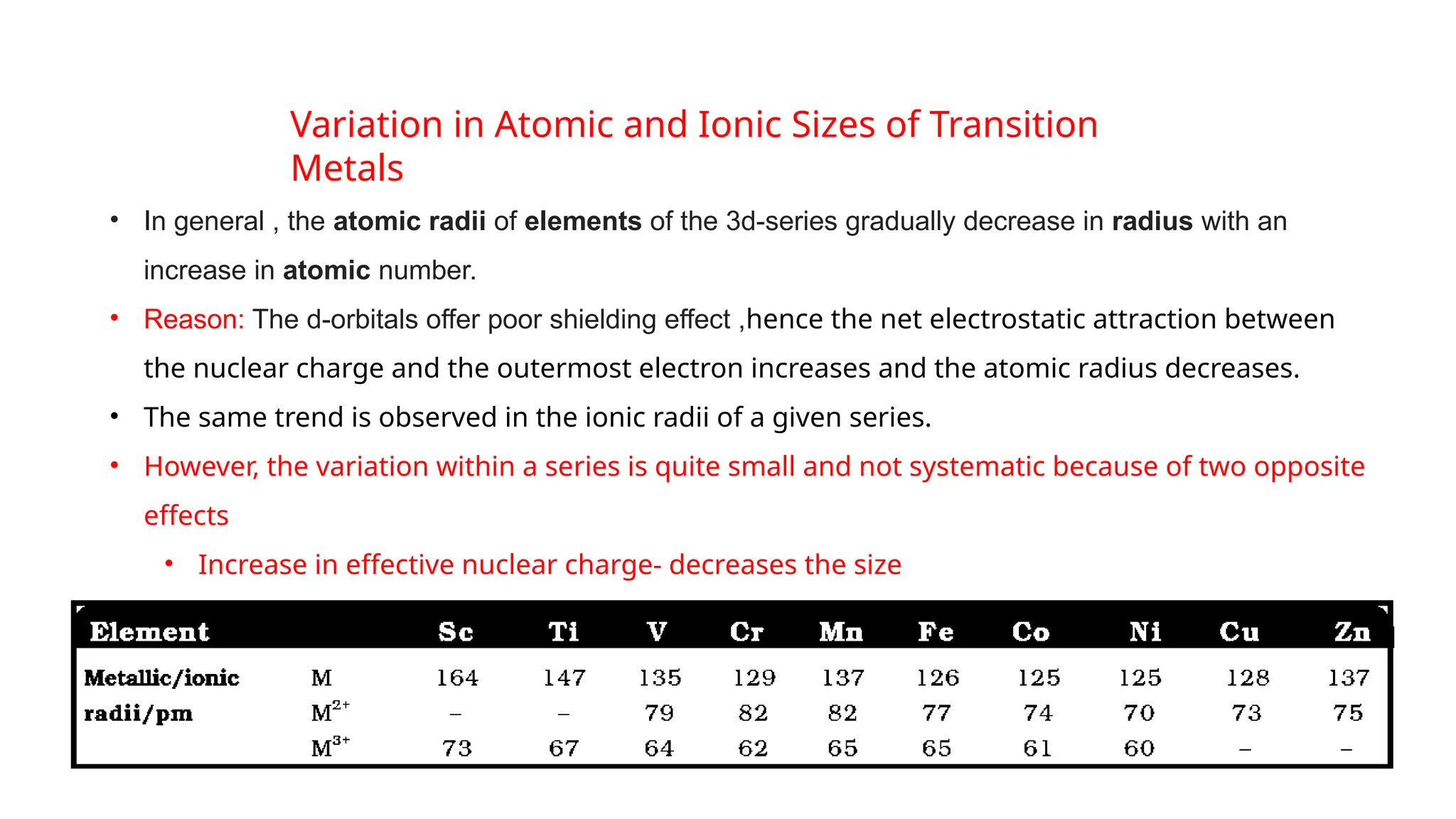
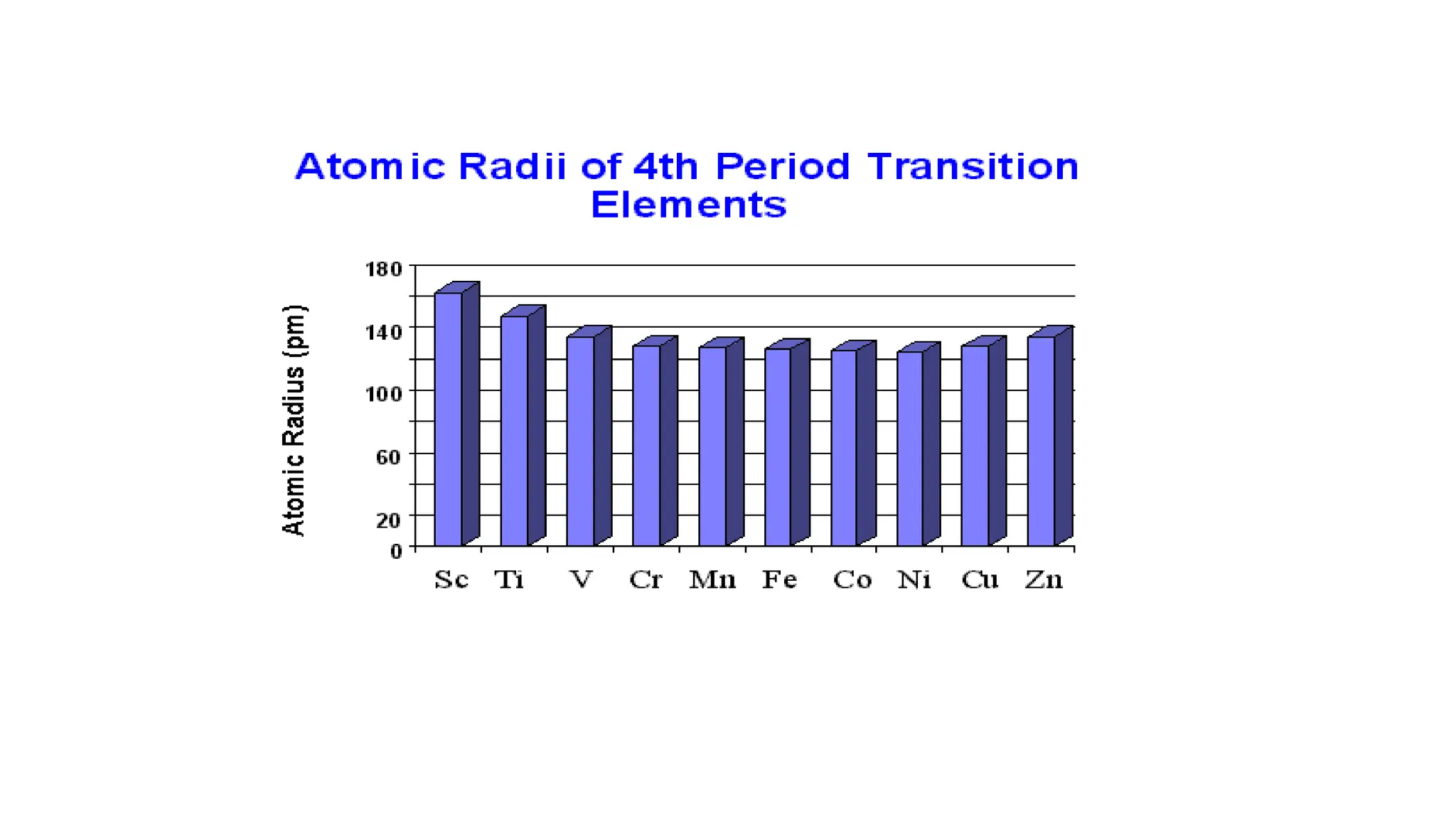
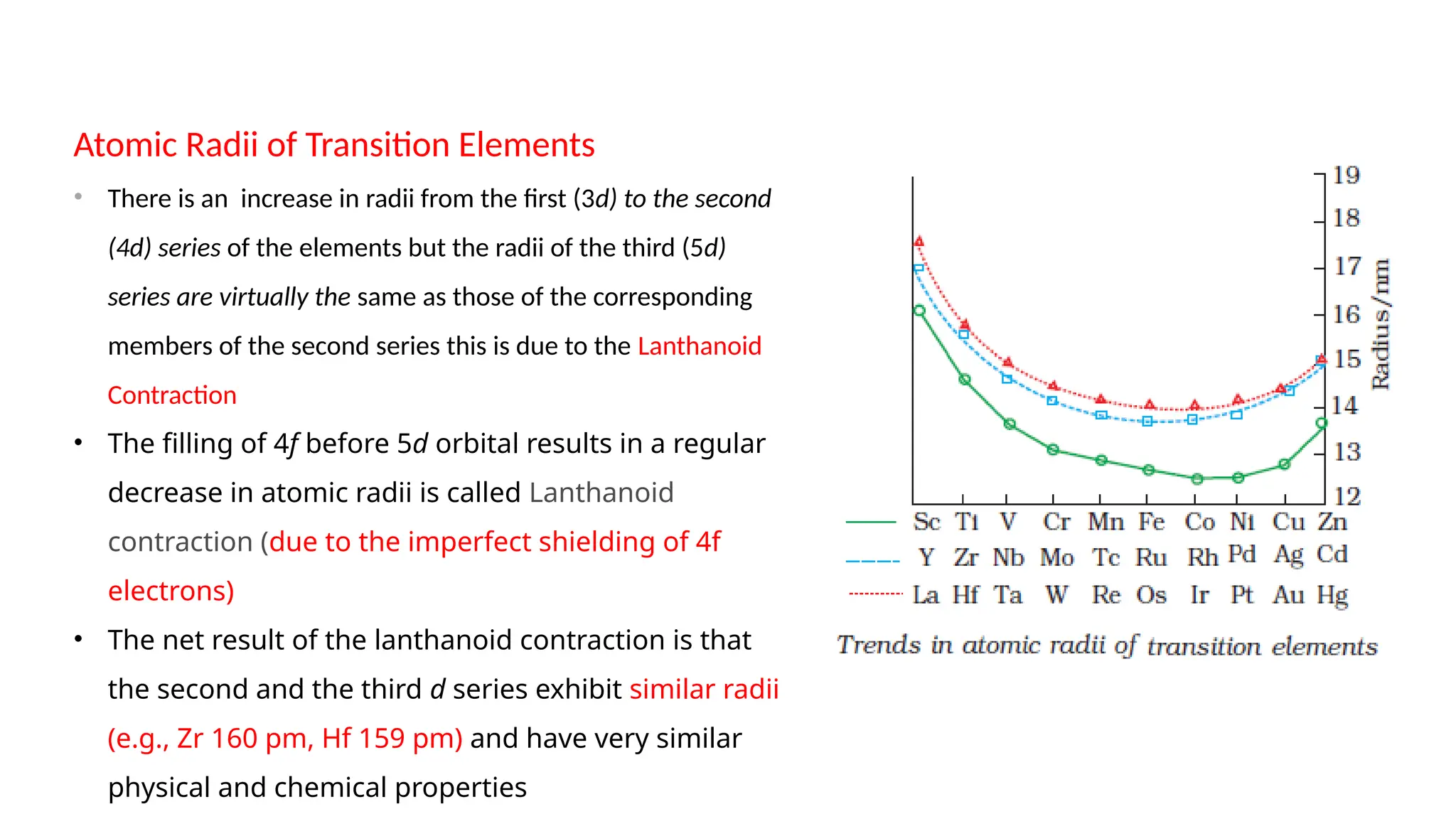
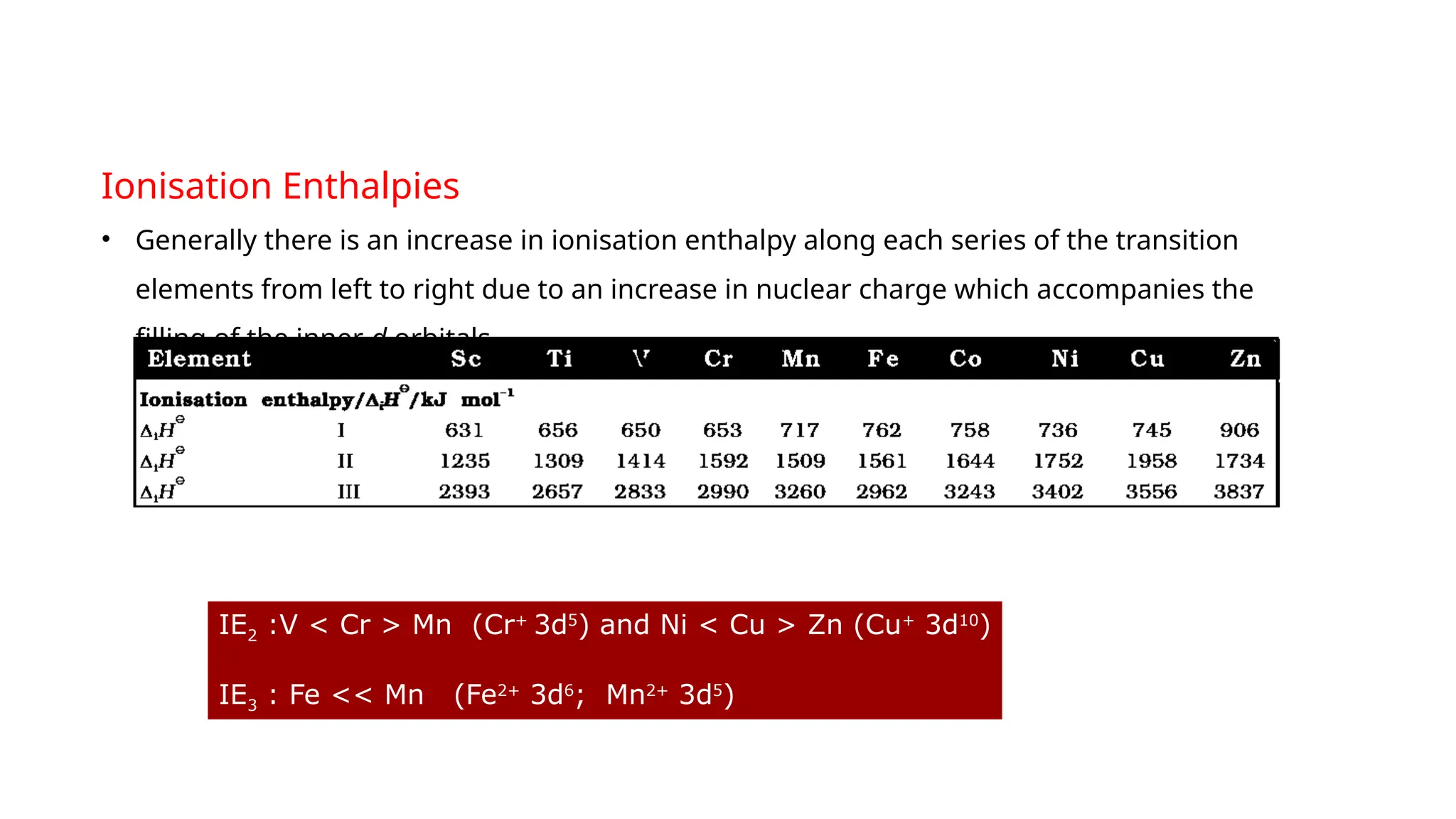
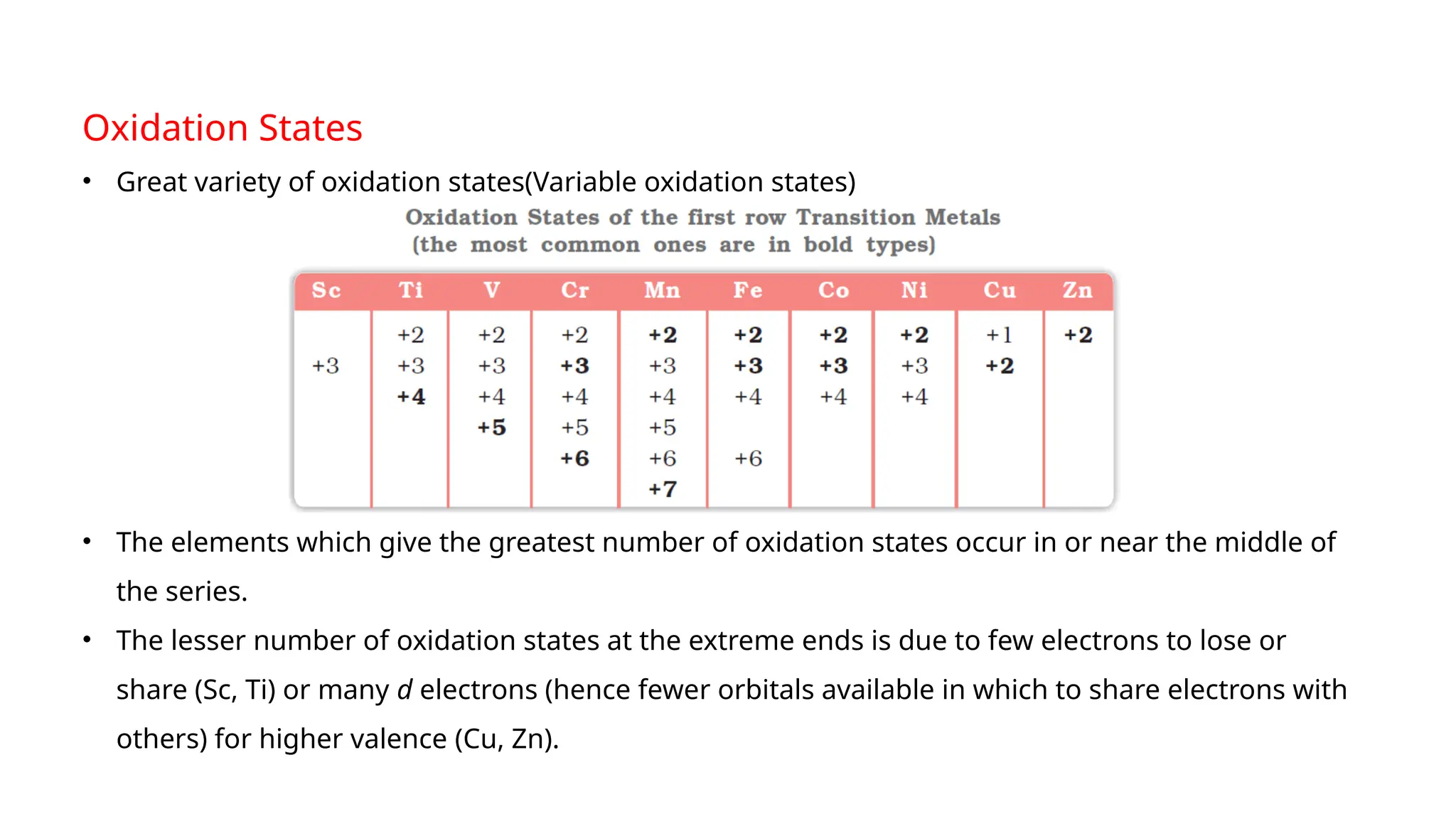
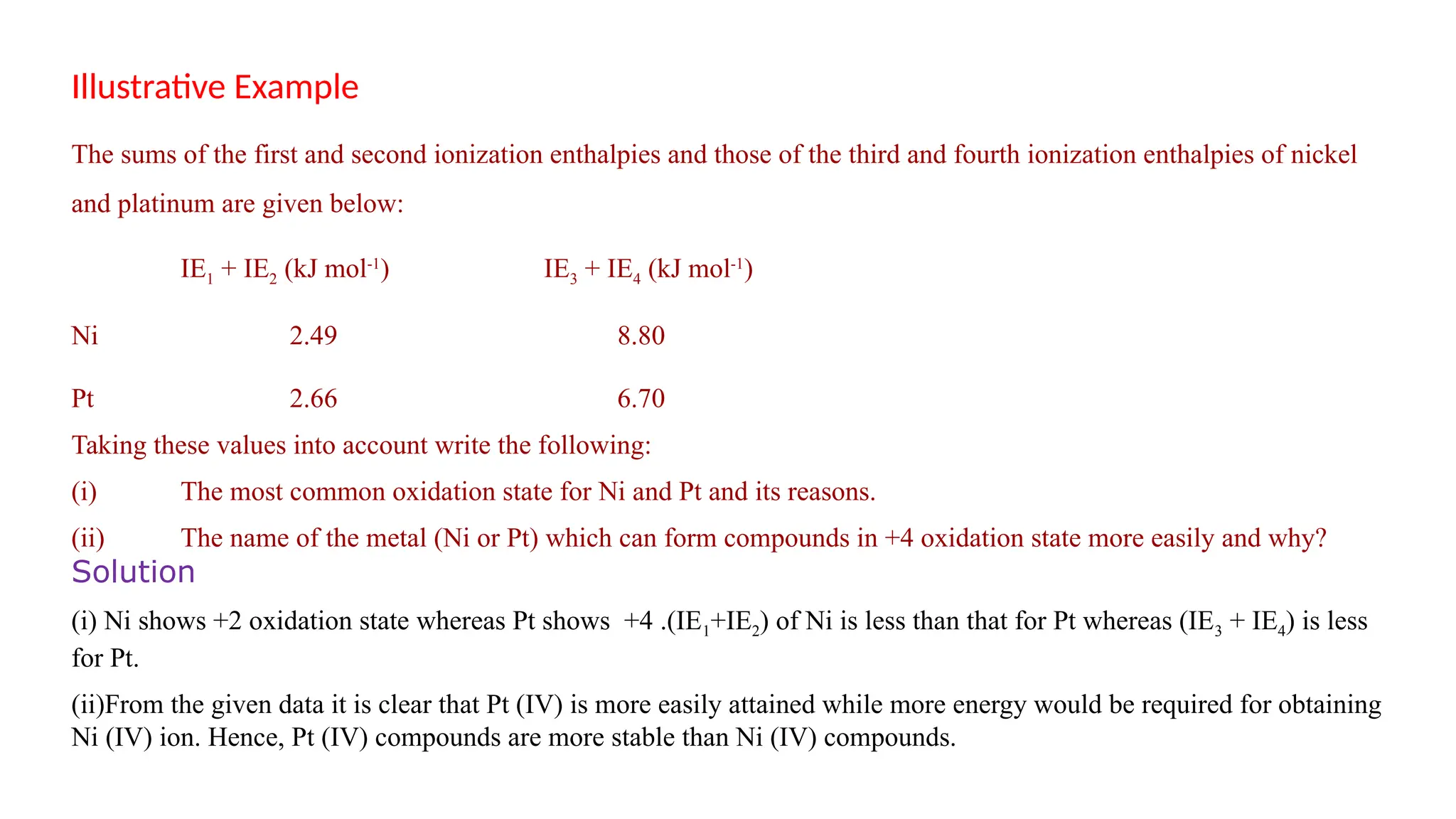
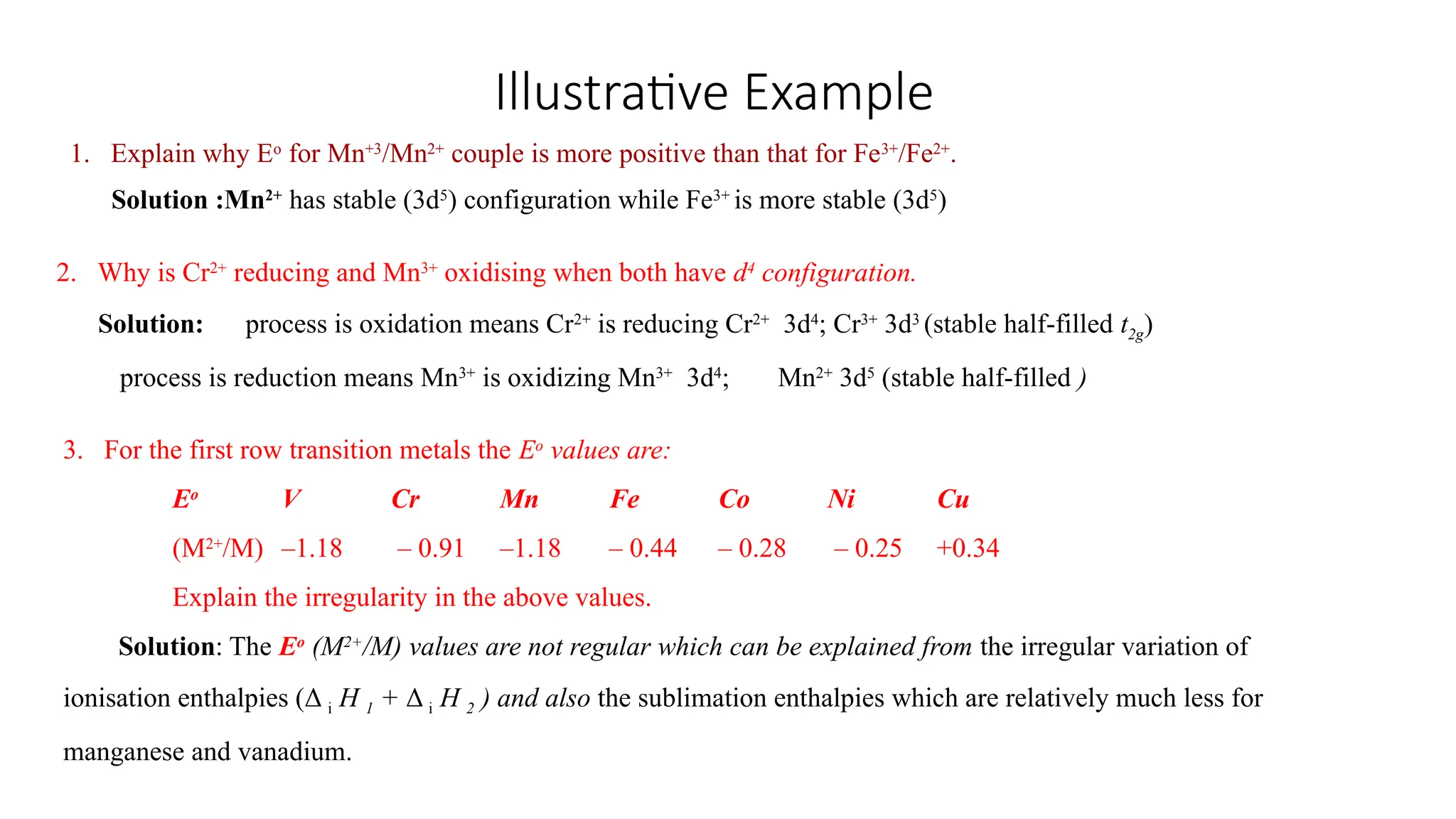
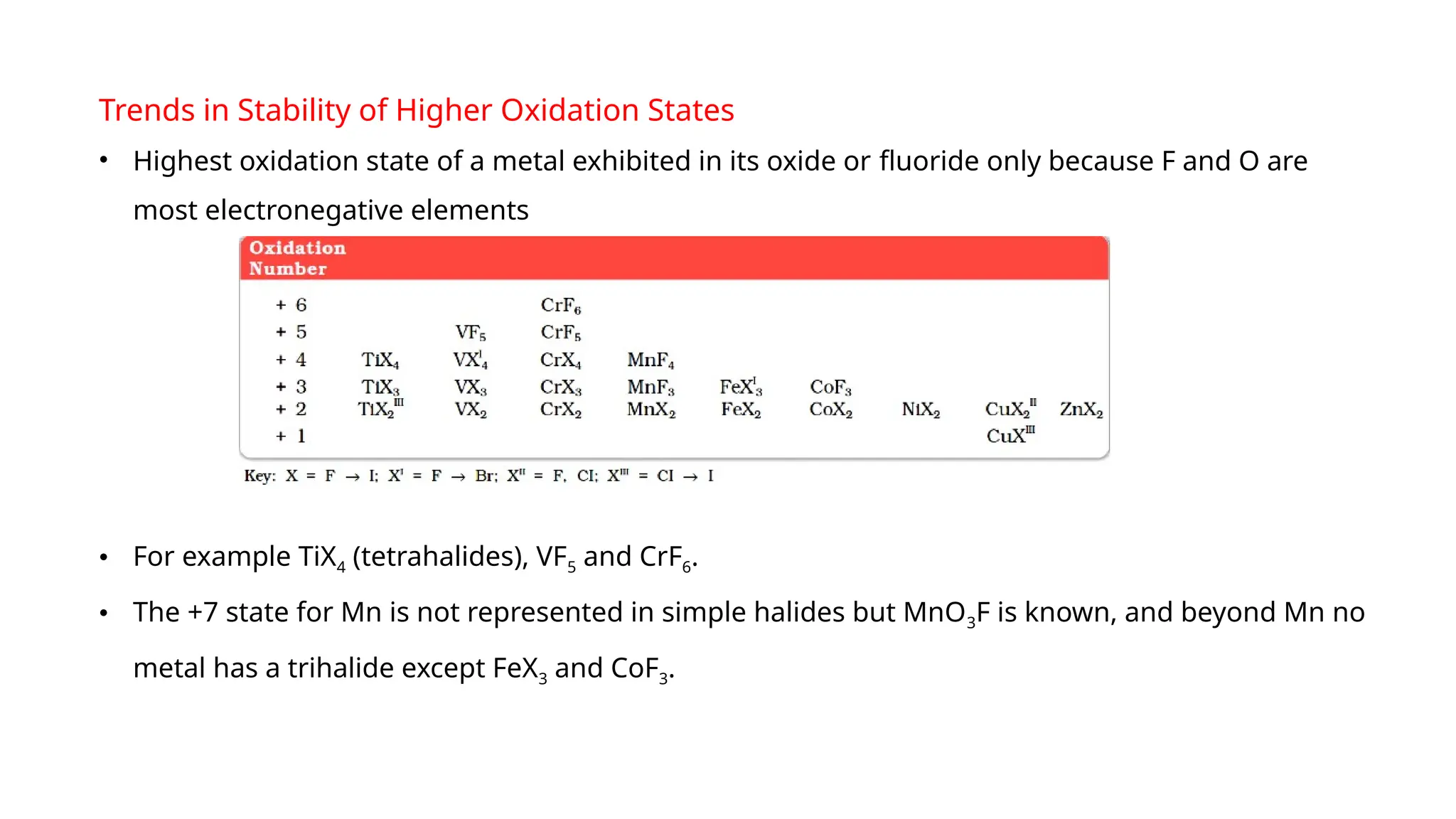
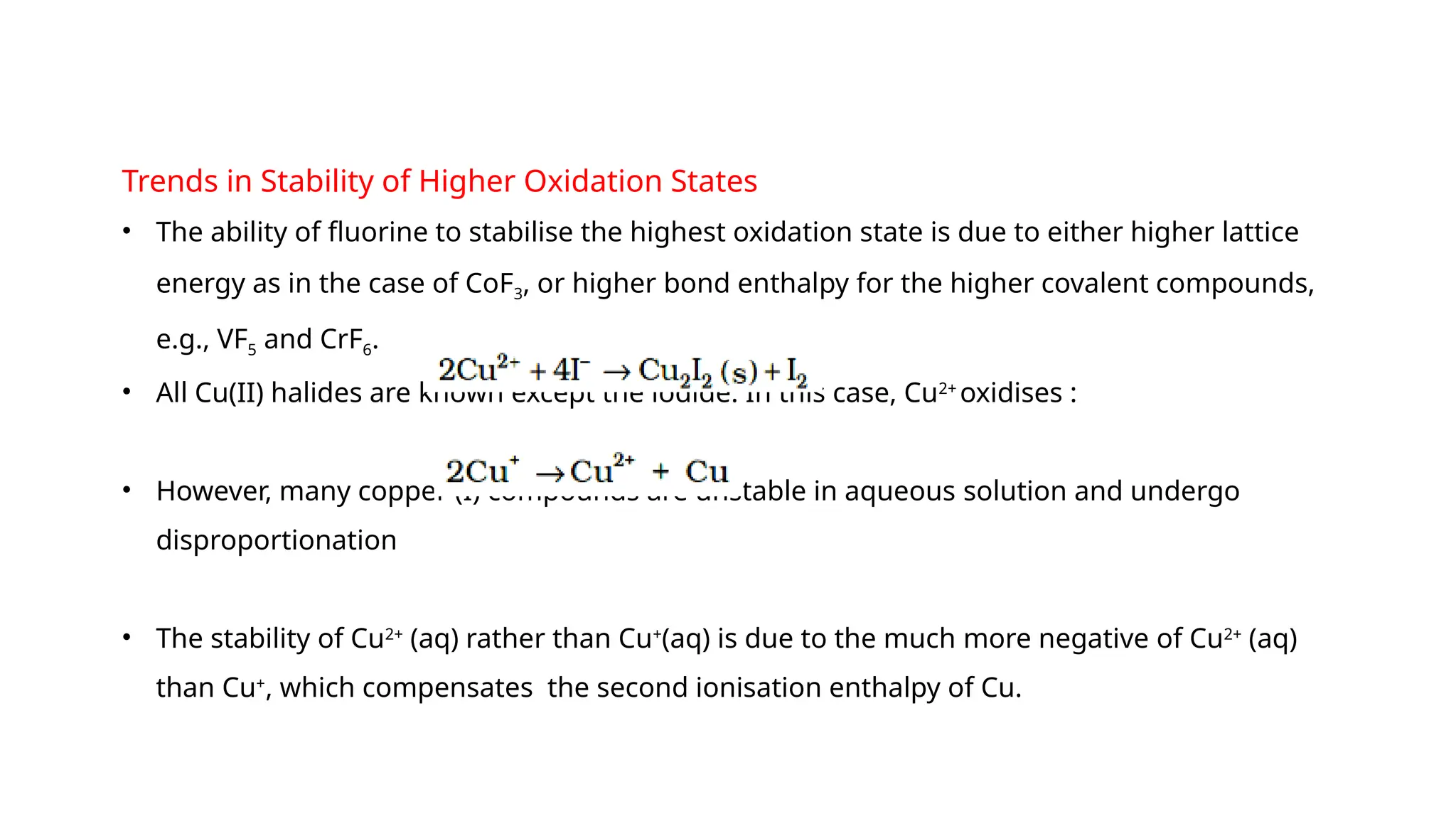
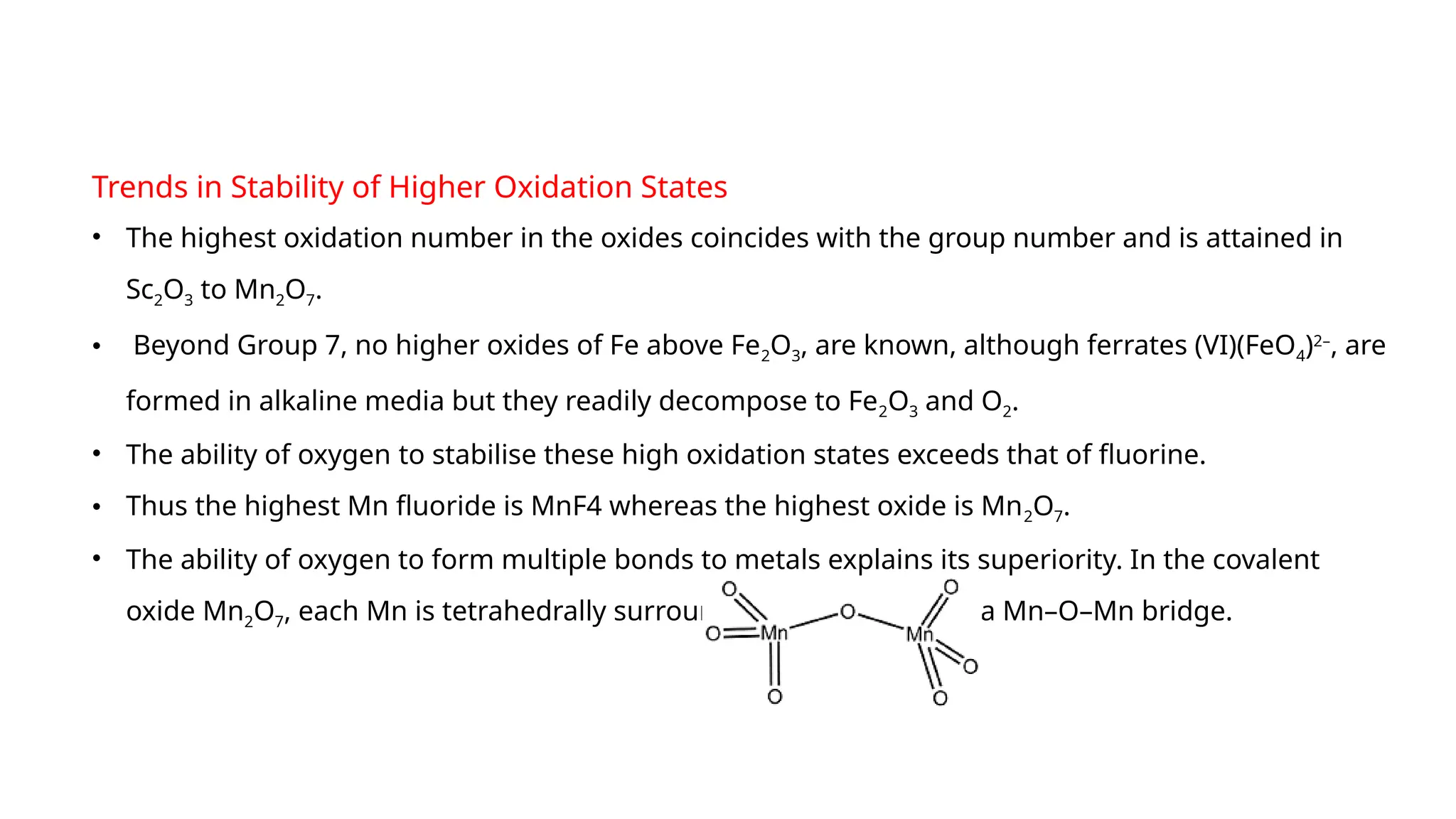
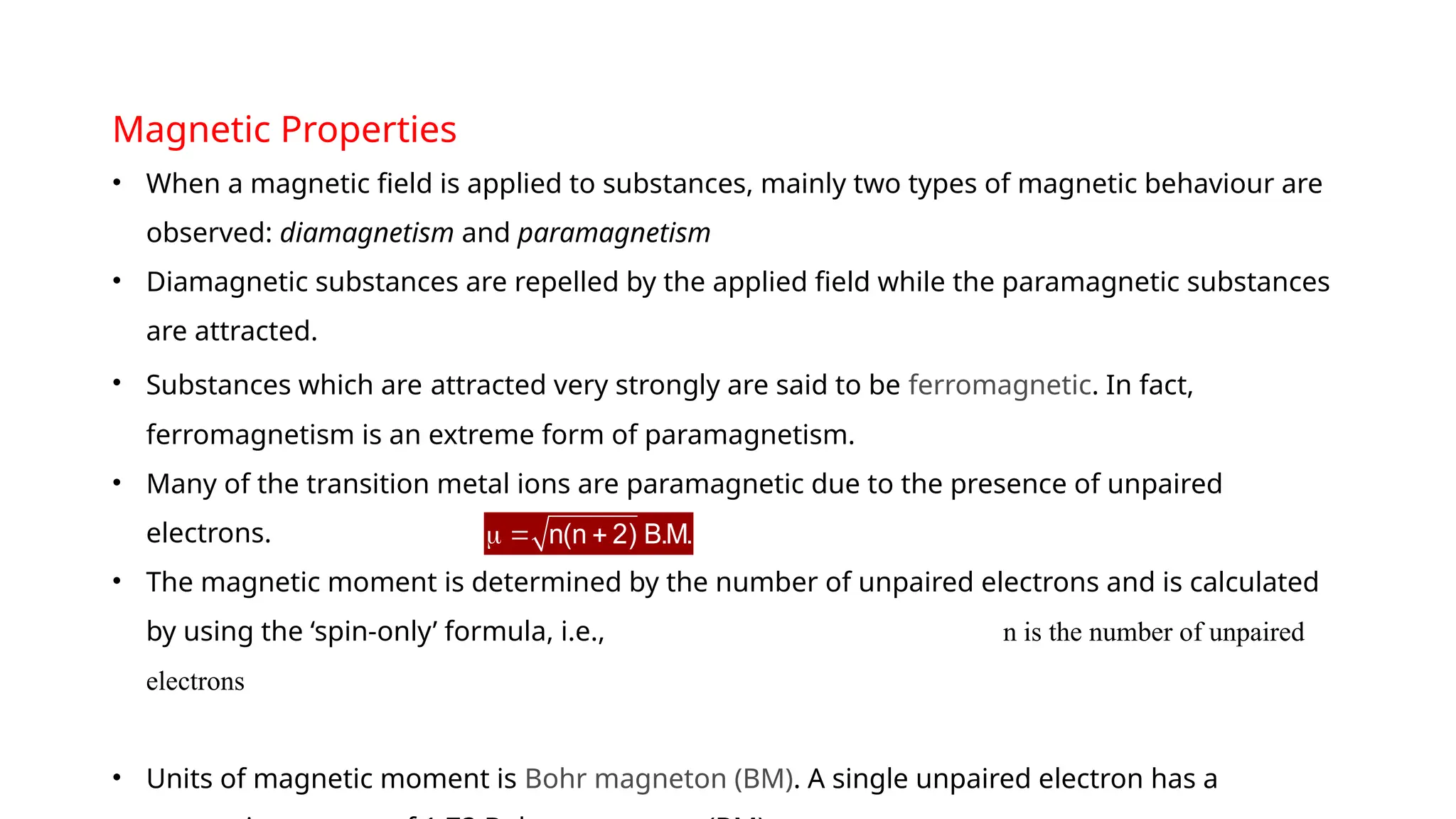
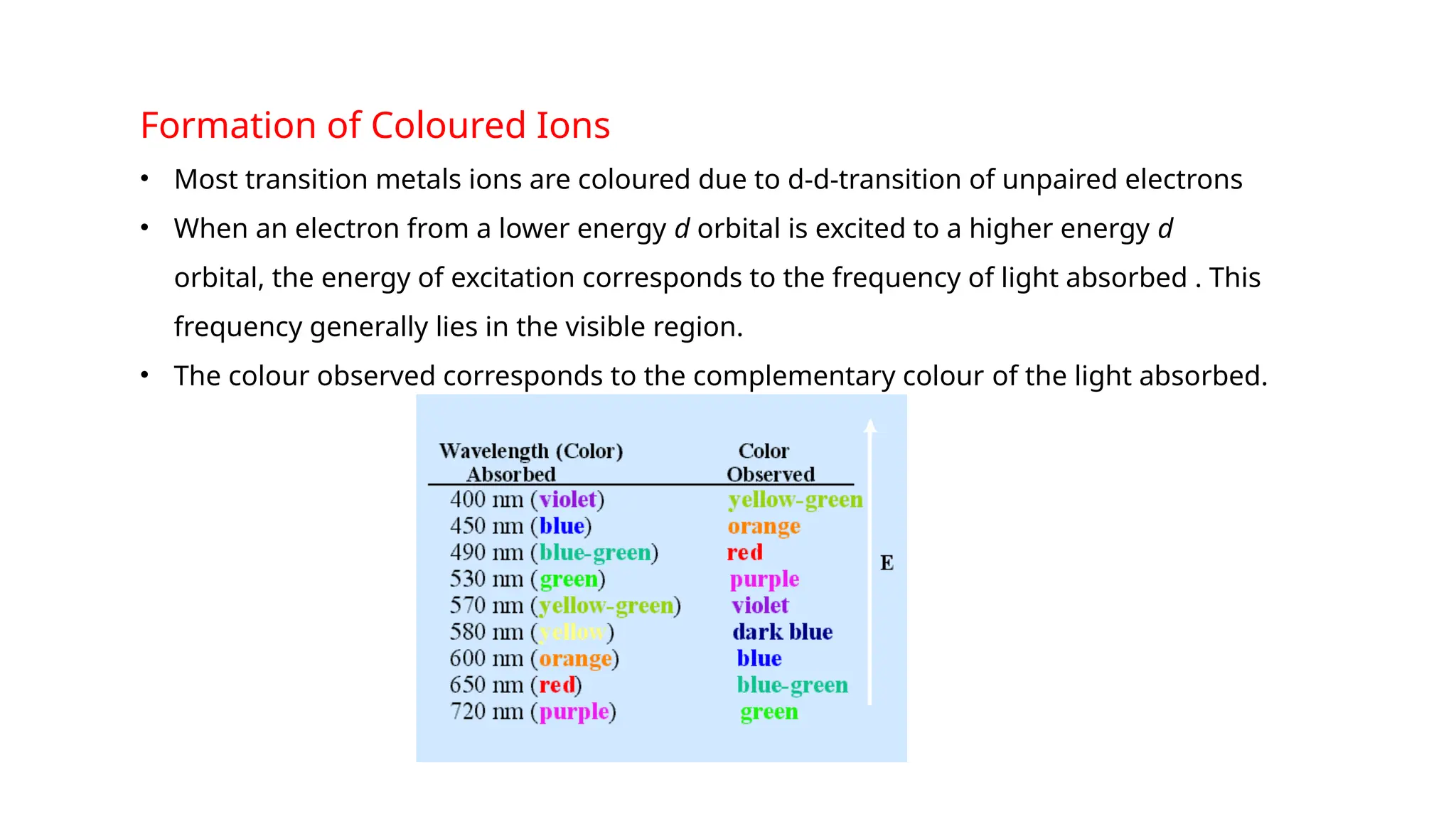
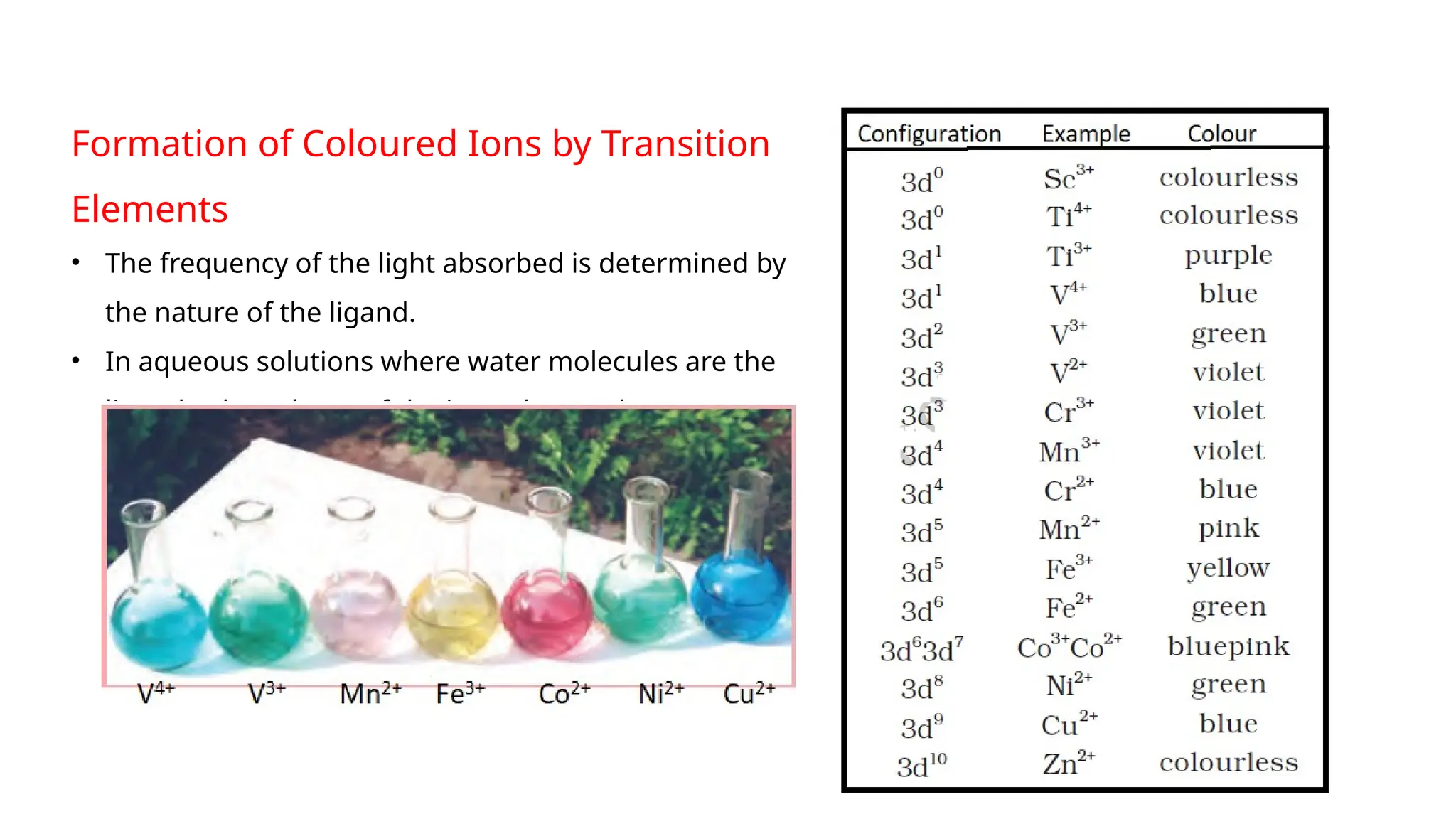
The document discusses the d- and f-block elements in chemistry, focusing on transition metals, their characteristics, and various properties such as electronic configurations, melting points, atomic sizes, ionization enthalpies, and oxidation states. It explains the significance of transition metals, their magnetic properties, colored ions, complex compounds, catalytic properties, and interstitial compounds. Additionally, it highlights trends in standard electrode potentials and the stability of oxidation states among transition metals.






![Formation of Complex Compounds
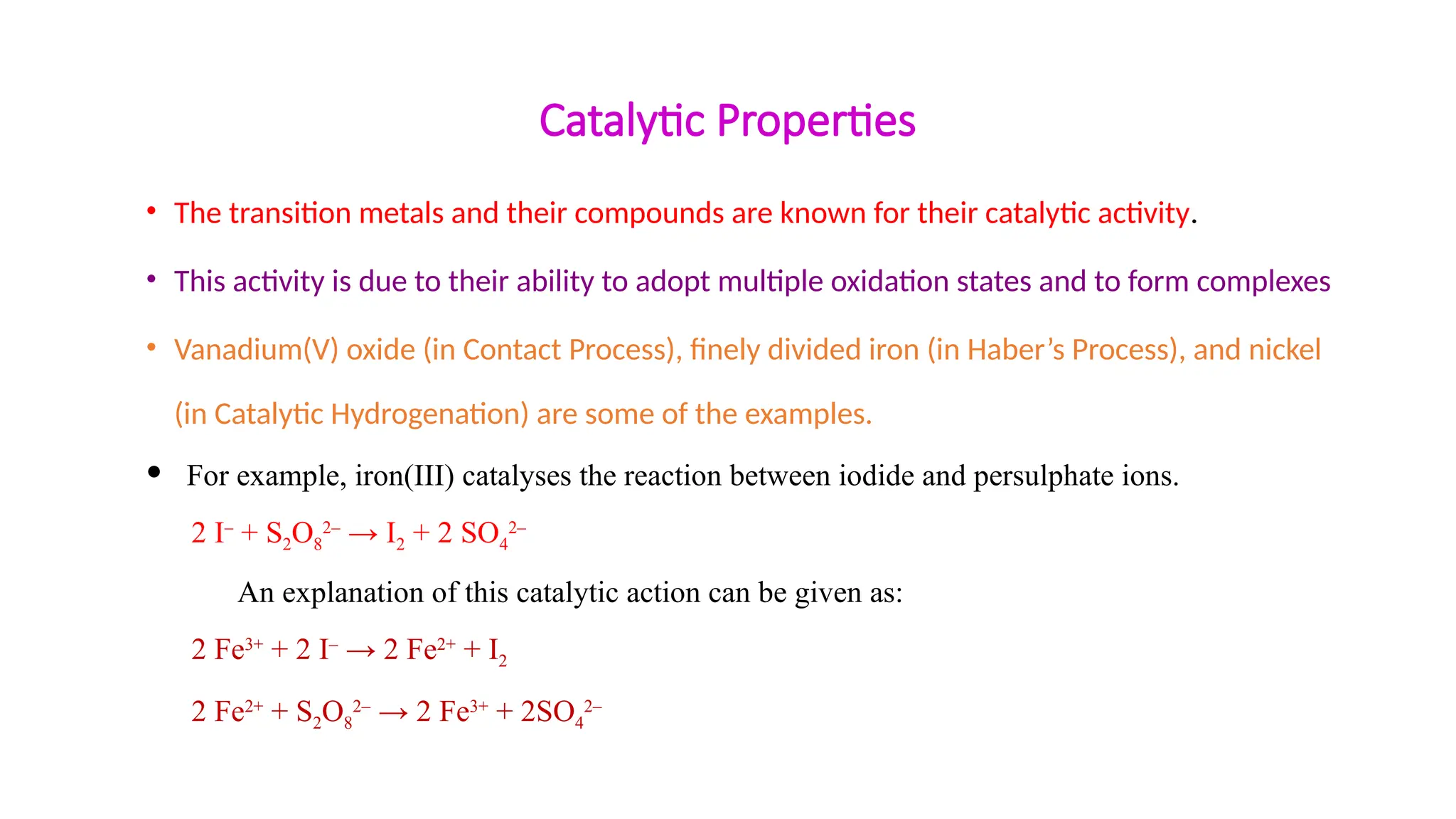
• The transition metals form a large number of complex compounds.
• This is due to the comparatively smaller sizes of the metal ions, their high ionic charges and the
availability of d orbitals for bond formation.
• A few examples are: [Fe(CN)6]3–
, [Fe(CN)6]4–
, [Cu(NH3)4]2+
and [PtCl4]2–
.
• Complex compounds are those in which the metal ions bind a number of anions or
neutral molecules giving complex species with characteristic properties.](https://image.slidesharecdn.com/class12chapter8thed-andf-blockelements-250115135138-c549261b/75/Class-12-Chapter-8-The-d-and-f-Block-Elements-pptx-33-2048.jpg)