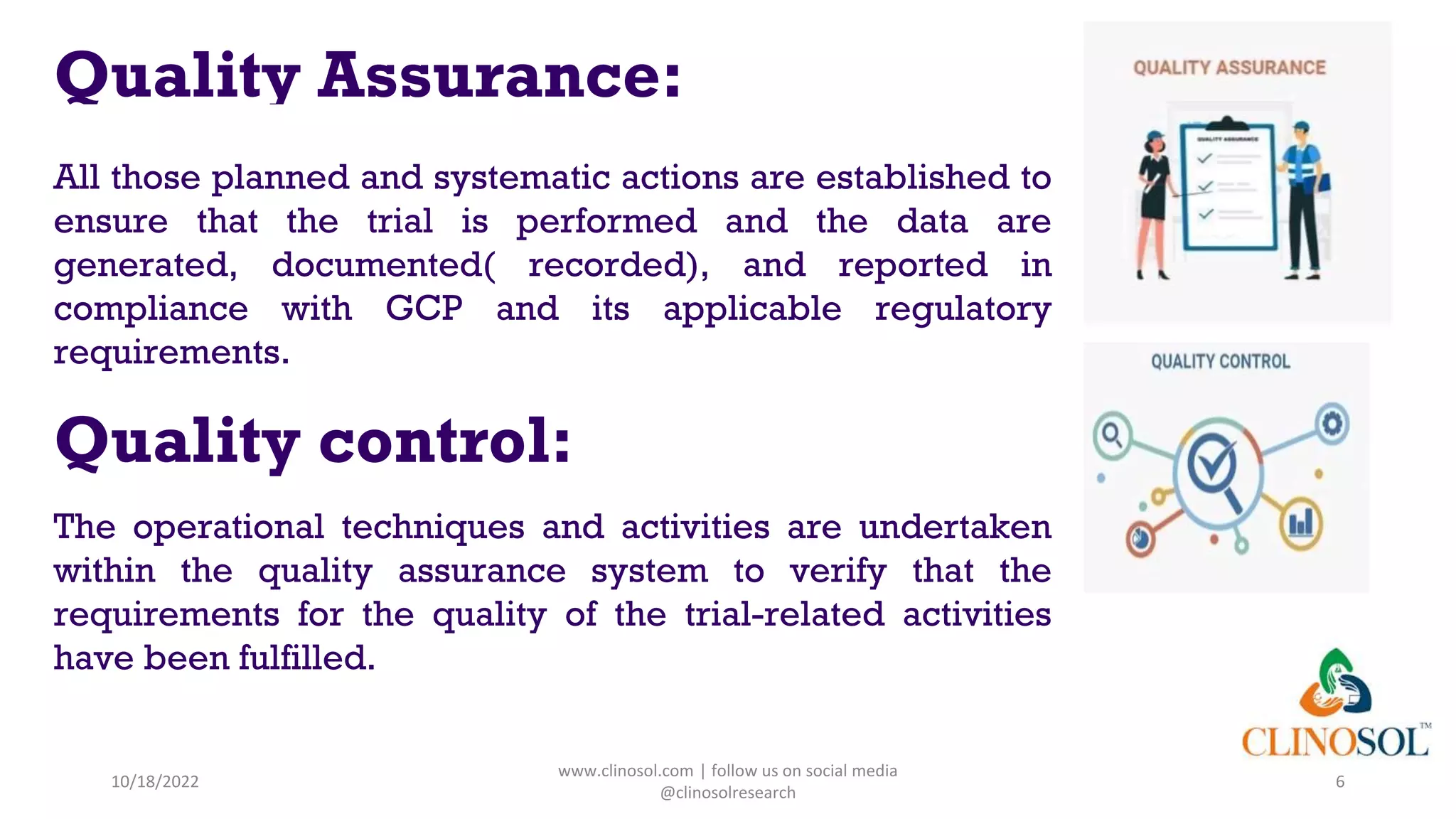
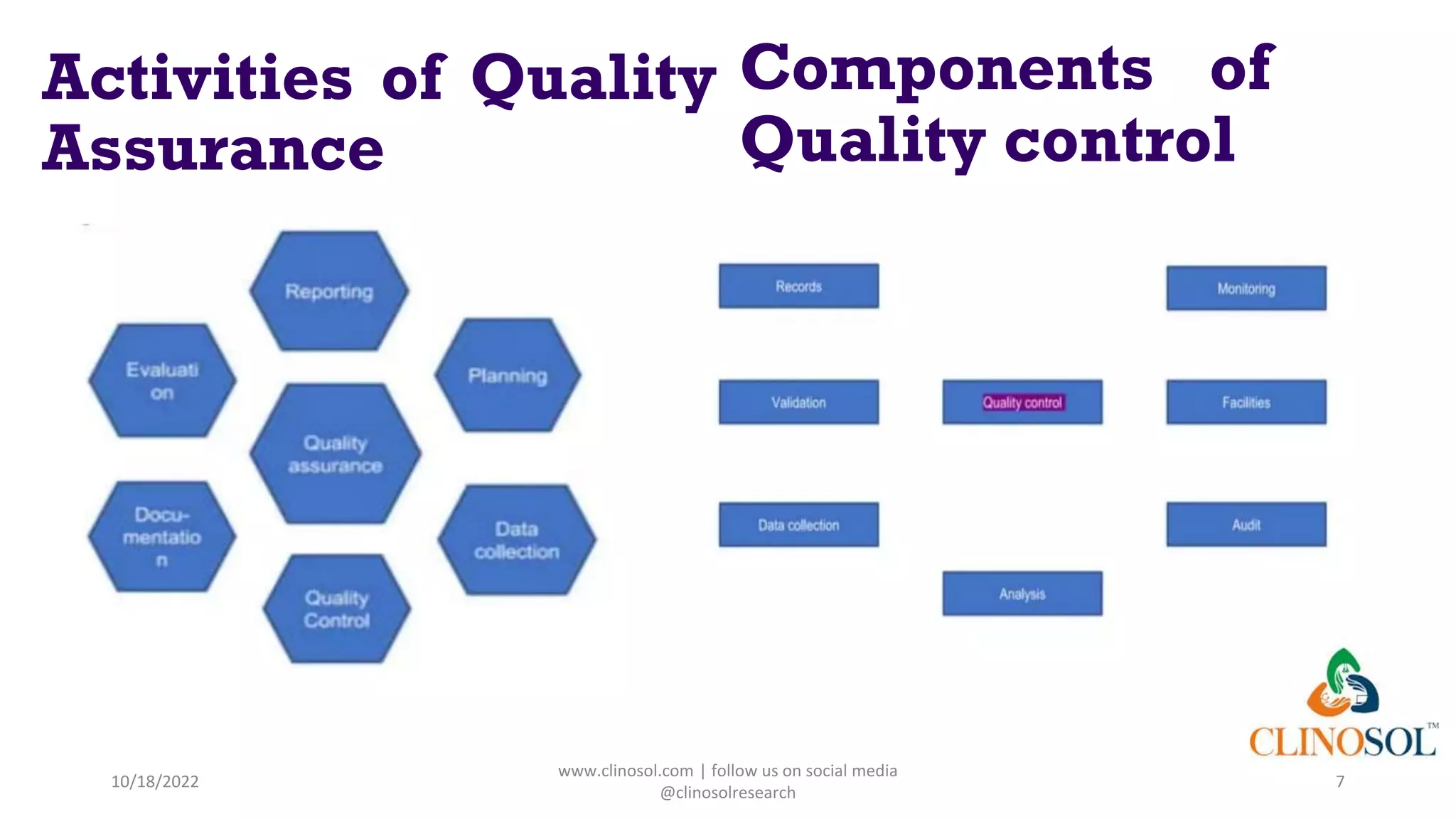
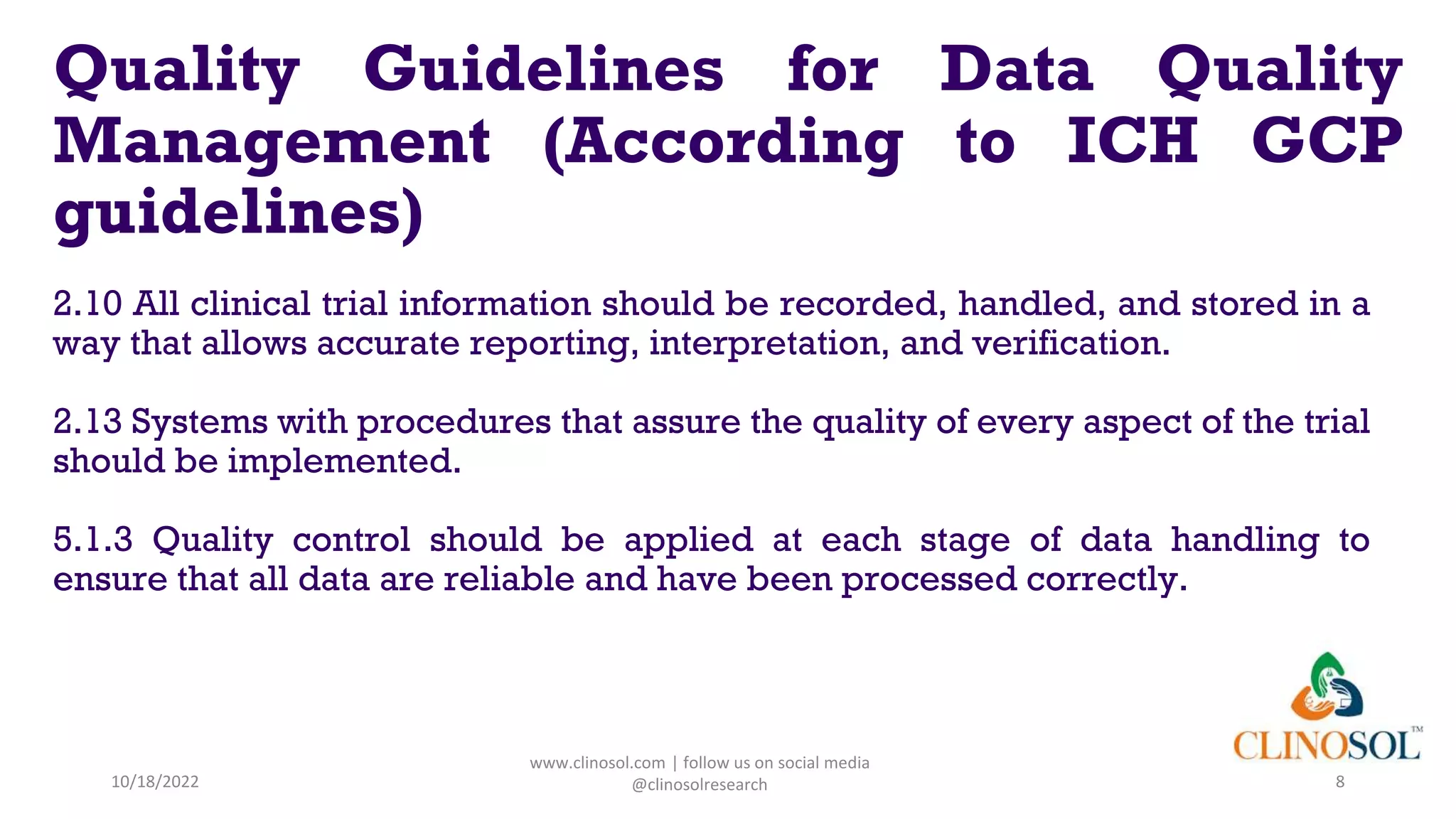
The document discusses data quality management in clinical trials, outlining its definition, components, and the importance of ensuring data integrity and accuracy. It highlights various responsibilities of sponsors and investigators, including adhering to guidelines and maintaining quality assurance and control. Key challenges in data quality management are also addressed, emphasizing the need for proper monitoring of data practices throughout the research process.





![The current Quality challenge in
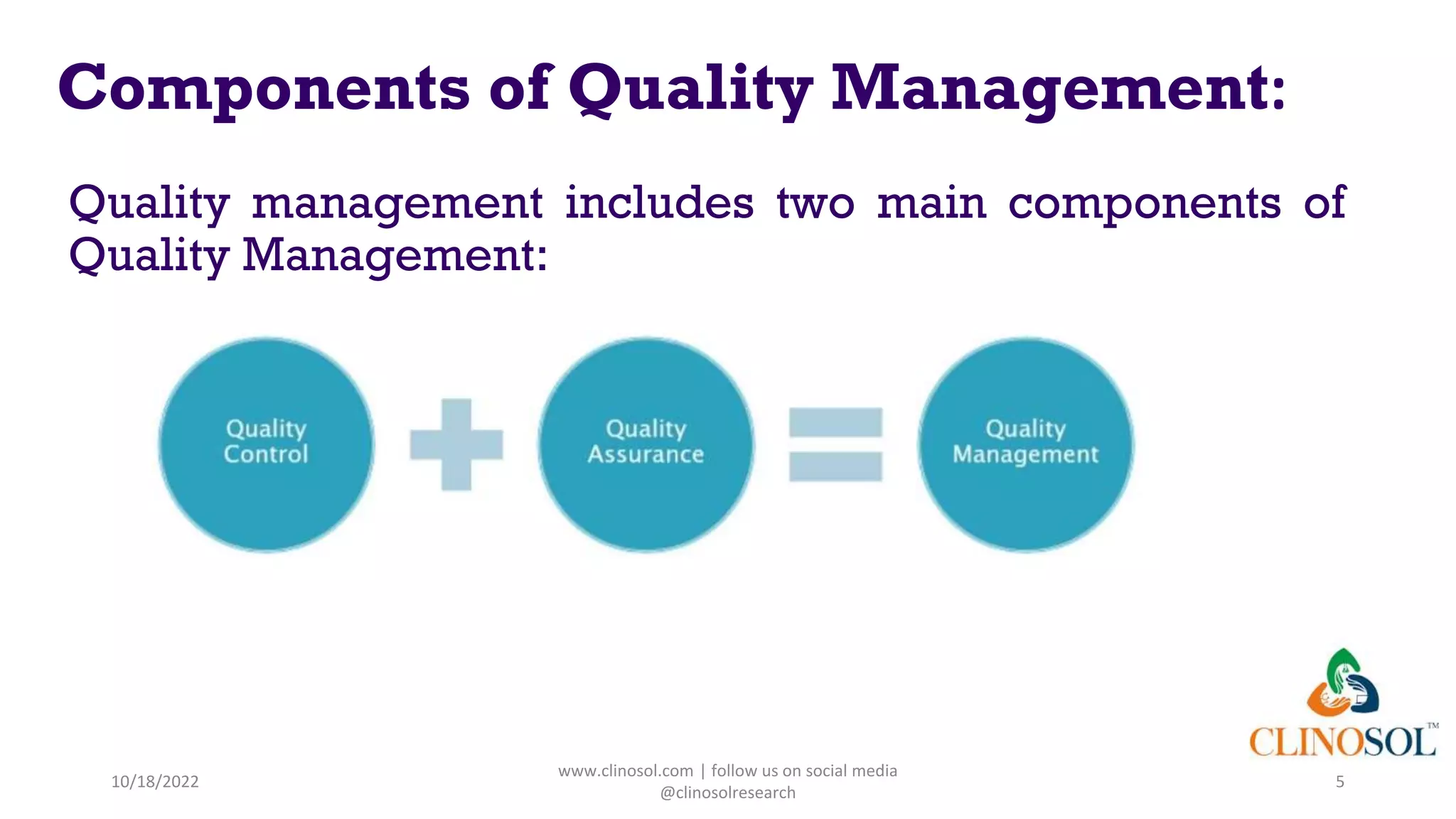
Data quality management:
• The ongoing challenge in managing the quality of clinical data is to
continually monitor data collection procedures and data management
practices at every level of the study. This includes:
• Ensuring that data generated during the study reflect what is specified
in the protocol (case report form[CRF] vs. protocol)
• Comparing data in the CRF and data collected in source documents for
accuracy (CRF vs. source documents).
10/18/2022
www.clinosol.com | follow us on social media
@clinosolresearch
11](https://image.slidesharecdn.com/doc-20230720-wa0015-230809201459-ec86a568/75/Data-Quality-Management-in-Clinical-Trials-11-2048.jpg)