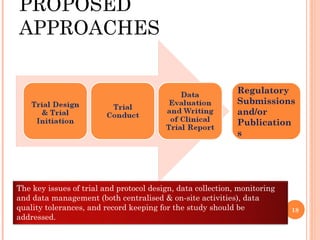
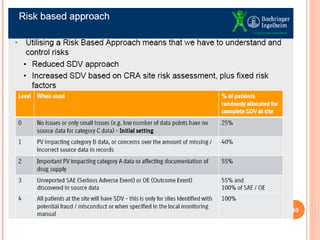
The document discusses quality assurance in clinical trials. It begins by outlining key frameworks for quality like GCP and ISO standards. It emphasizes that quality assurance requires understanding critical parameters, incorporating quality by design principles, and addressing practical challenges in data collection and documentation. The document then provides examples of risk-based quality management, highlighting the importance of identifying, assessing, and mitigating risks at various stages of a clinical trial's lifecycle. Finally, it presents two case studies, one on a large heart failure trial and another on a global respiratory trial, to demonstrate operationalizing quality assurance practices.






![WHAT QUALITY & AT WHAT
COST?
The most pragmatic definition of quality is “fitness
for purpose”
Simply striving for the “highest level” of quality has
little practical meaning
Current practices: either success at an [unnecessarily]
high cost or failure also very costly
A practical & cost effective paradigm:
“Adequate quality of a CT should be such that the
decisions made would have been no different had the
quality of data and information generated been perfect”
EMA reflection paper on risk based quality management in clinical trials 8](https://image.slidesharecdn.com/qcinclinicaltrialsfinal-121104223727-phpapp01/85/Qc-in-clinical-trials-8-320.jpg)

![THE CURRENT QUALITY
CHALLENGE
The ongoing challenge in managing the quality of clinical data is to
continually monitor data collection procedures and data management
practices at every level of the study. This includes:
Ensuring that data generated during the study reflect what is specified
in the protocol (case report form[CRF] vs. protocol)
Comparing data in the CRF and data collected in source documents for
accuracy (CRF vs. source documents)
Ensuring that the data analyzed are the data recorded in the CRF
(database vs. CRF).Quality surveillance continues after the trial has
ended and plays an important role in ensuring that:
Data presented in tables, listings, and graphs (TLGs) correctly match
data in the database (TLGs vs. database)
Data reported in the clinical study report (CSR) are the data analyzed
(CSR vs. TLGs)
All aspects of the data management processes are compliant with SOPs
and GCPs. 10](https://image.slidesharecdn.com/qcinclinicaltrialsfinal-121104223727-phpapp01/85/Qc-in-clinical-trials-10-320.jpg)

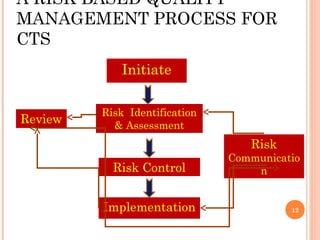
![RISK BASED QUALITY
MANAGEMENT
Key idea & practice:
Identify, assess, control & mitigate, communicate,
and review [both risks & remedies]
All risks [low, mid & high grade] associated with the
clinical trial during its lifecycle
RBQM facilitates better and more informed
decision making and makes best use of the
available resources
Should be appropriately documented and
integrated within existing quality systems
Responsibility of all involved parties to
contribute to the delivery of an effective RBQM
13](https://image.slidesharecdn.com/qcinclinicaltrialsfinal-121104223727-phpapp01/85/Qc-in-clinical-trials-13-320.jpg)