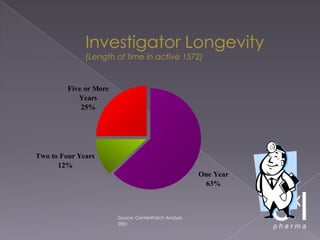
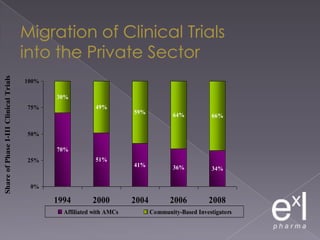
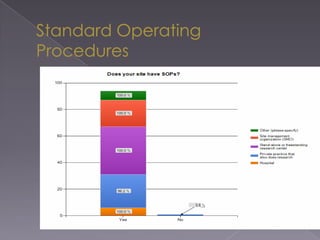
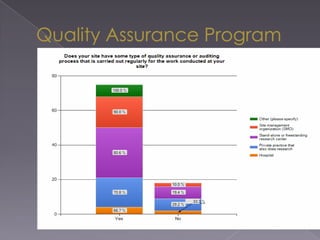
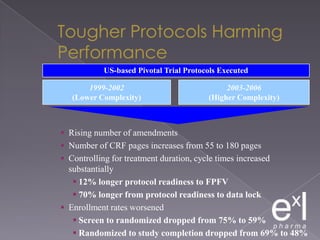
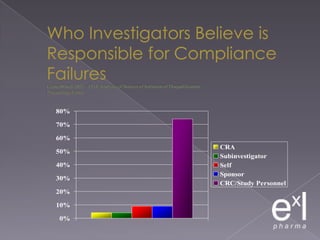
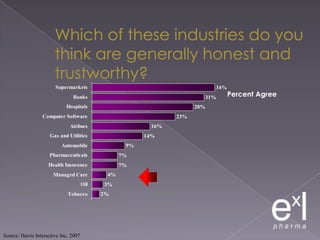
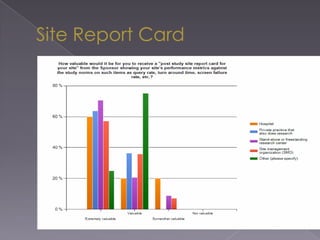
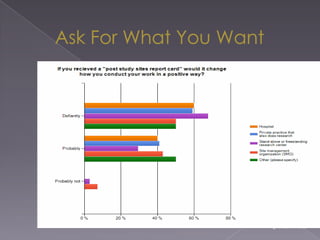
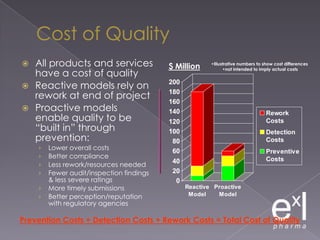
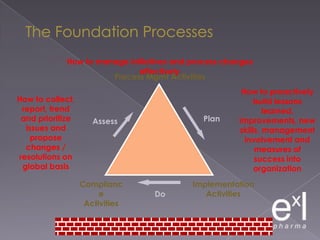
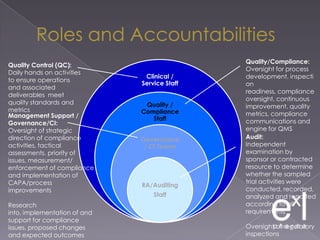
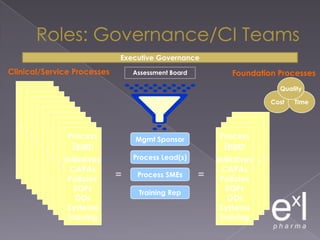
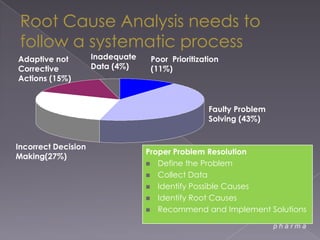
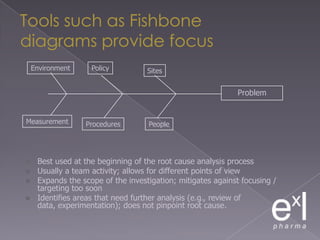
This document summarizes a conference on Good Clinical Practice (GCP) compliance. It discusses the objectives of clinical research and challenges to harmonizing GCP standards internationally. It also outlines current ethical challenges in clinical trials and dimensions of GCP frameworks. The document proposes that further developing national and regional GCP guidance, broadening the scope of GCP, and establishing appropriate platforms can help advance GCP. It provides guidance on investigator responsibilities and comments on adverse event reporting.