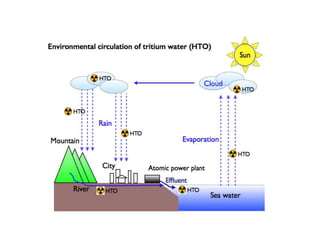
Tritium occurs naturally and is produced anthropogenically. It exists as tritiated water, a radioactive form of water also known as tritium oxide or super-heavy water. Tritium is found globally in air, water sources, and oceans due to natural processes and human activities like nuclear weapons testing. While naturally occurring tritium concentrations are very low, anthropogenic sources from nuclear testing injected tritium into the stratosphere which then entered surface waters and oceans. Present day tritium levels vary by location, with higher concentrations in northern hemisphere waters and lower levels in the southern oceans. Tritium water has similar physical properties to ordinary water but can cause health effects if exposed including cancer and genetic defects.
![Natural tritium concentrations in water:
• tritium concentrations are reported as TU
• 10-18 tritium atoms per hydrogen atom.
• a tritium to hydrogen ratio [T]/[H] of 10-18](https://image.slidesharecdn.com/hydrologypresentation-170429093541/85/Tritium-present-in-water-5-320.jpg)