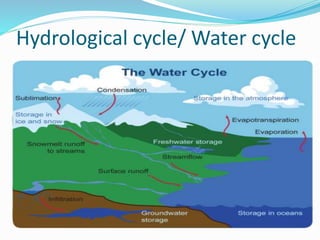
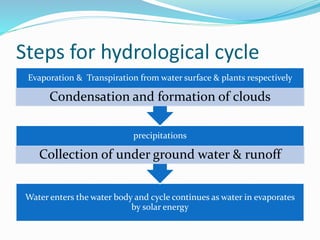
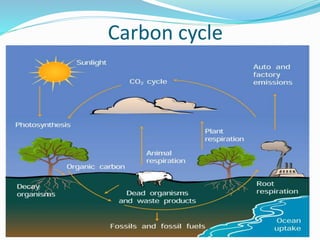
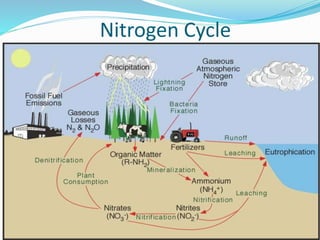
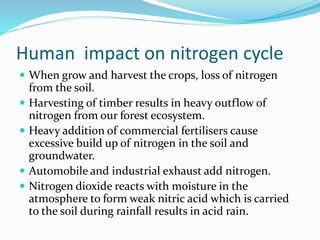
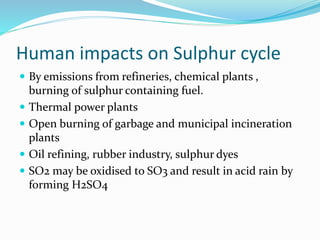
The document summarizes several biogeochemical cycles. It describes the carbon, nitrogen, water, and sulfur cycles. For each cycle it explains the sources, pathways, and how elements are exchanged between living organisms and the environment. It also discusses some human impacts such as burning fossil fuels, deforestation, fertilizer use, and industrial emissions which can disrupt the natural cycling of these elements.