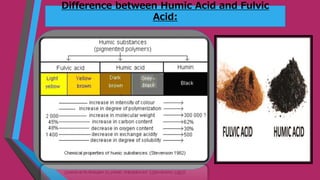
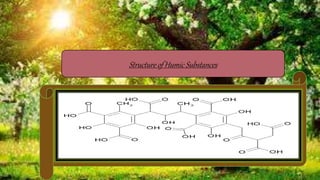
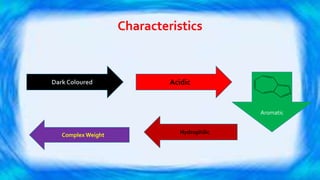
Humic substances are major components of natural organic matter found in soils, sediments, and geologic deposits. They are formed by the microbial degradation of dead plant matter. Humic substances can be divided into humic acid, fulvic acid, and humin, and are characterized by their dark color, acidic and aromatic properties. They can complex with metals in natural waters and transport them long distances. Their ability to form complexes influences coagulant dosing in water treatment. Humic substances can impact human health, with humic acid possibly forming disinfection byproducts during chlorination, while fulvic acid may have benefits for brain health and infections but also impacts allergic reactions.