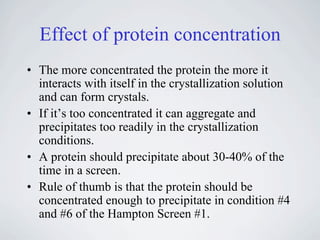
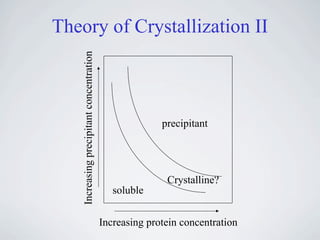
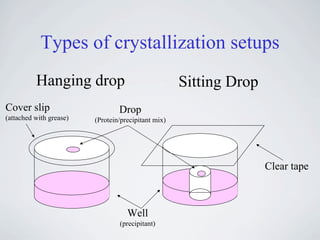
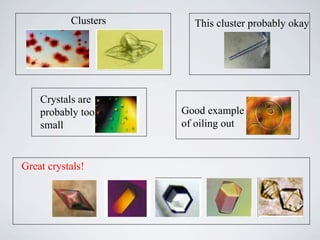
The document provides information on protein crystallization, including what is needed to crystallize a protein, how to improve crystallization chances, the theory behind crystallization setups and conditions, evaluating crystallization screens, and obtaining and analyzing protein crystals. The key aspects are having a pure and concentrated protein, screening a variety of conditions to find initial "hits", and optimizing these conditions through small changes to precipitants and additives to obtain high-quality crystals.