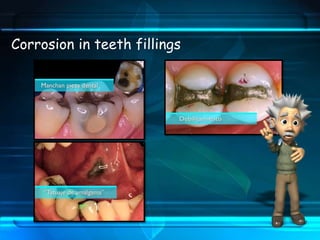
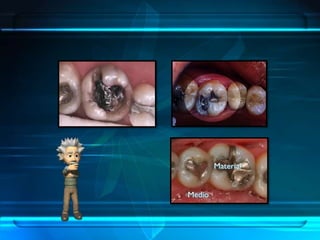
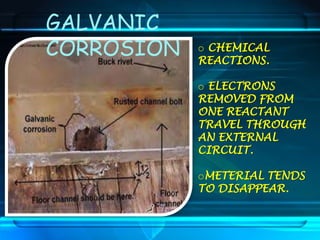
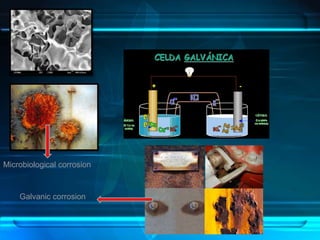
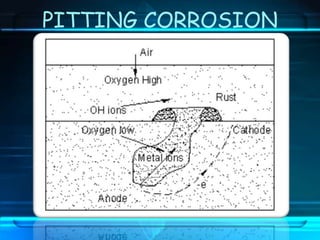
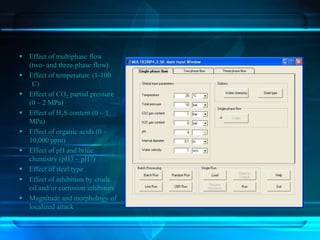
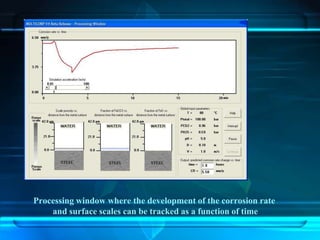
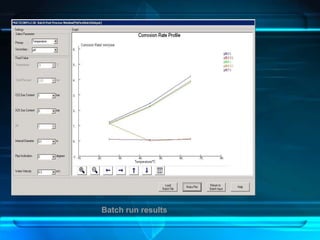
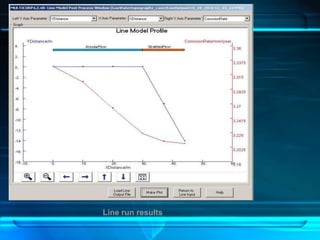
This document discusses corrosion of engineering materials and methods to prevent it. It provides background on corrosion, noting that all materials will corrode to some degree depending on their environment. Factors like temperature, pressure, and harmful gases can cause corrosion analogous to their effects on the human body. The document then covers different types of corrosion like uniform, galvanic, pitting, stress, and microbiological corrosion. It discusses the economic impacts of corrosion and methods to prevent it, including coatings, inhibitors, cathodic protection, and controlling factors like pH. Software models for predicting corrosion rates are also summarized, such as Multicorp, PREDICT, and Norsok M-506.