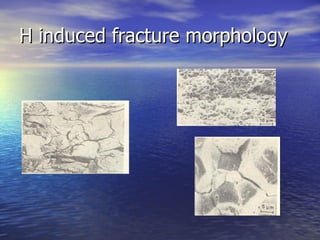
The document discusses the detrimental effects of hydrogen on metallic materials, including various forms of hydrogen damage such as embrittlement and cracking. It details the mechanisms through which hydrogen interacts with metals, the conditions that exacerbate the damage, and methods for prevention. The findings underscore the importance of materials selection and processing to mitigate hydrogen-related damage in structural materials.