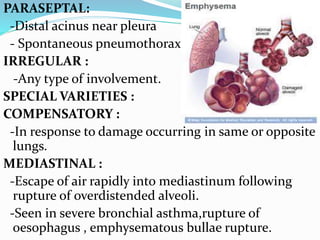
This document provides an overview of chronic obstructive pulmonary disease (COPD), including its definition, risk factors, pathophysiology, clinical presentation, diagnosis, complications, and treatment. COPD is characterized by persistent airflow limitation that is usually progressive. The major risk factor is cigarette smoking. Clinical features include cough, sputum production, and exertional dyspnea. Diagnosis is confirmed by spirometry showing reduced FEV1/FVC ratio. Treatment involves smoking cessation, bronchodilators, inhaled corticosteroids, pulmonary rehabilitation, oxygen therapy, and management of exacerbations with bronchodilators and antibiotics.