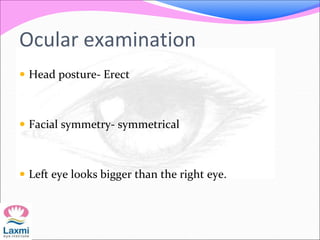
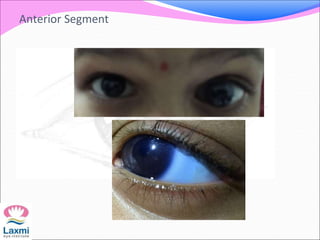
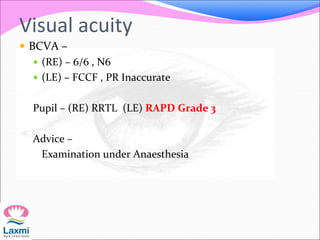
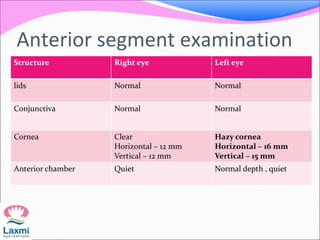
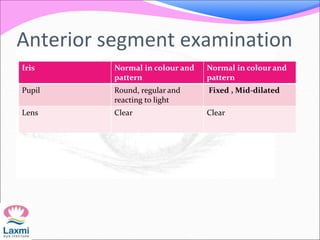
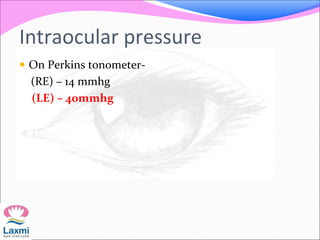
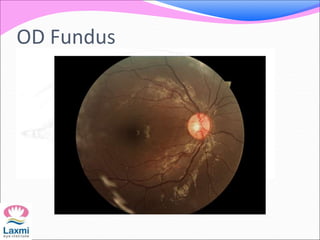
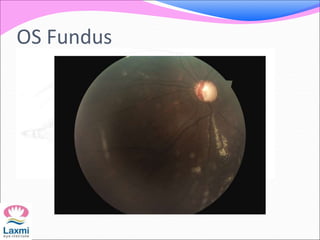
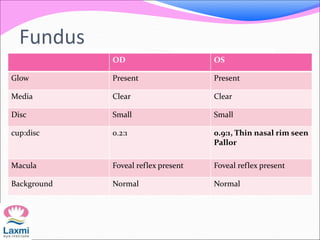
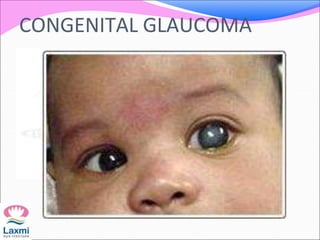
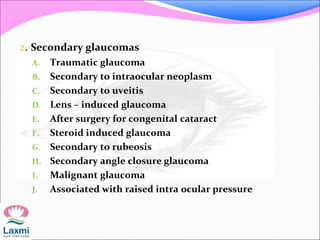
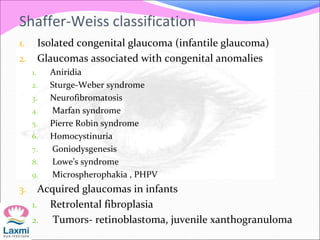
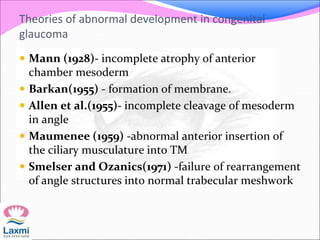
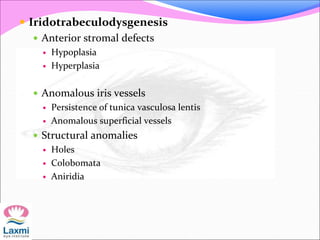
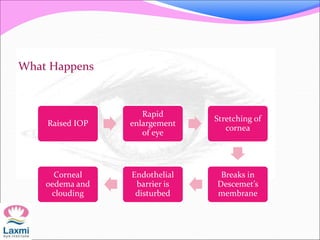
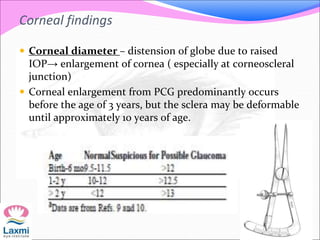
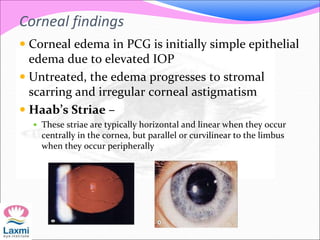
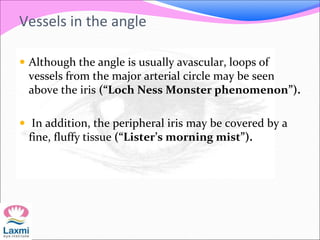
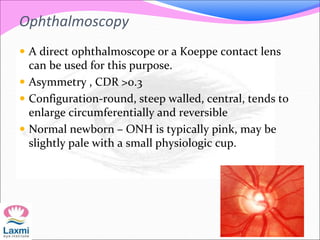
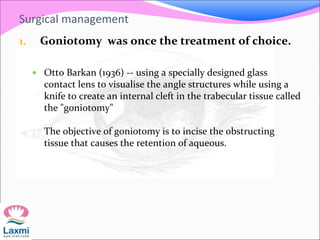
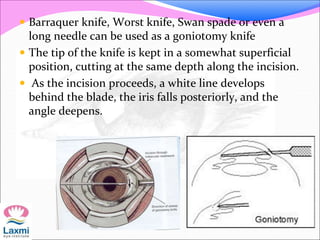
This document presents a case of congenital glaucoma in an 8-year-old female. Examination under anesthesia revealed increased intraocular pressure and a hazy cornea in the left eye, while the right eye was normal. A provisional diagnosis of congenital glaucoma in the left eye was made. Medical treatment was initiated but surgery was declined. The patient was followed up regularly with good intraocular pressure control on medication. The document then reviews definitions, epidemiology, genetics, classifications, theories of development and clinical features of congenital glaucoma.