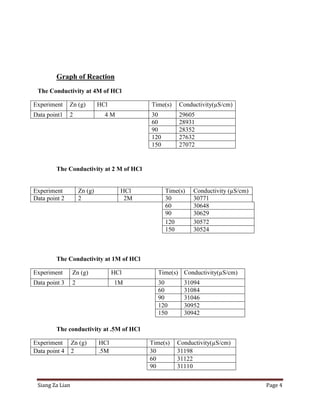
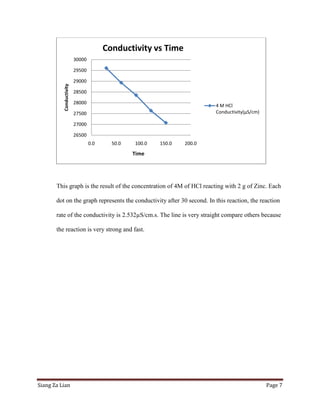
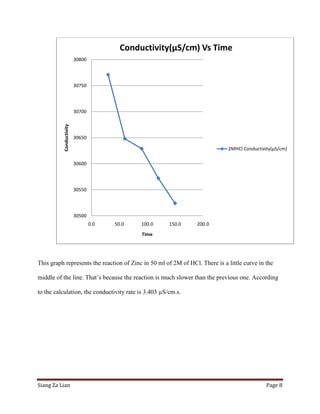
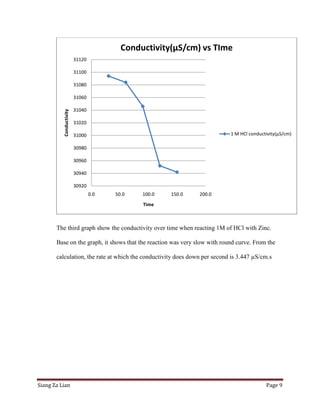
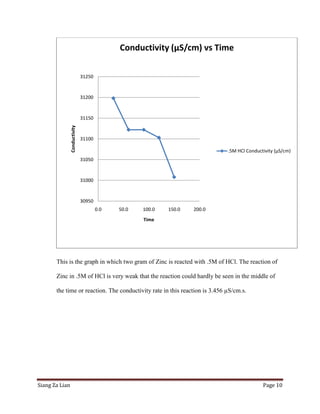
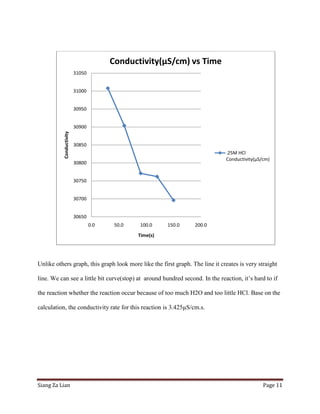
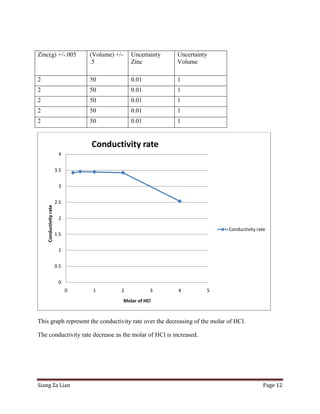
This experiment investigates how the concentration of hydrochloric acid (HCl) affects the conductivity when zinc is added. Five trials were conducted with varying molar concentrations of HCl (4M, 2M, 1M, 0.5M, and 0.25M) and the conductivity was measured over time. The results show that conductivity decreases more slowly at lower HCl concentrations. Specifically, the conductivity rate decreases from 2.532 μS/cm/s for 4M HCl to 3.425 μS/cm/s for 0.25M HCl. Thus, lower HCl concentrations lead to smaller decreases in conductivity over time when zinc is added.