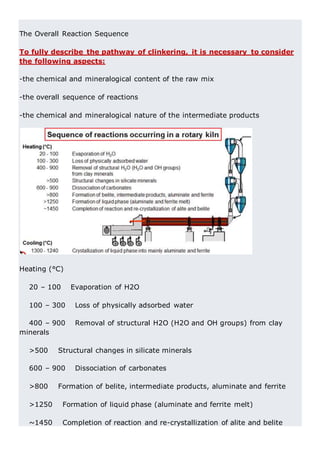
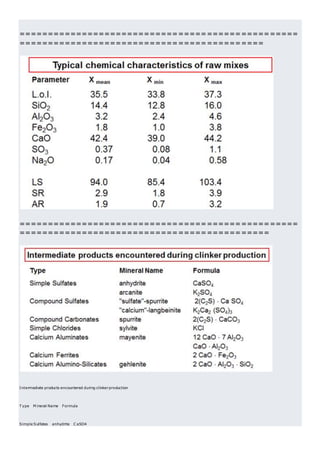
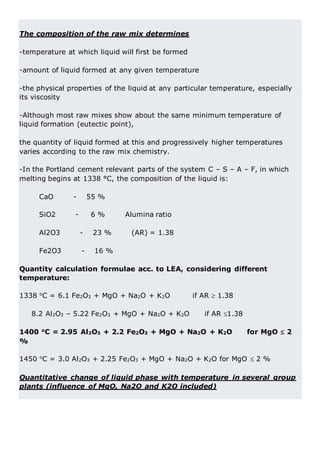
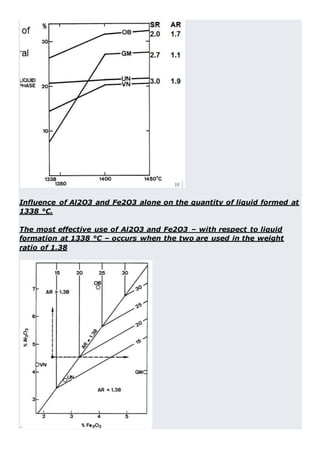
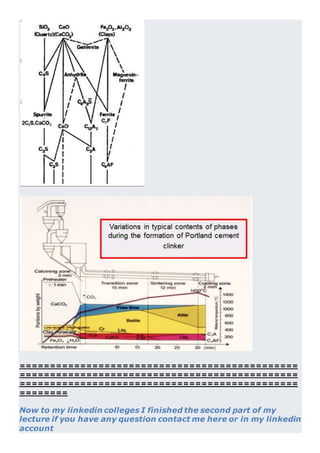
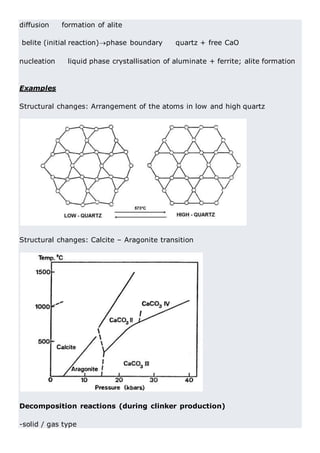
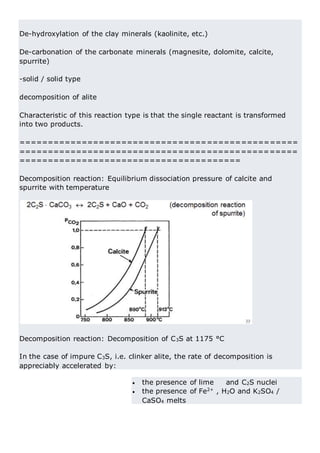
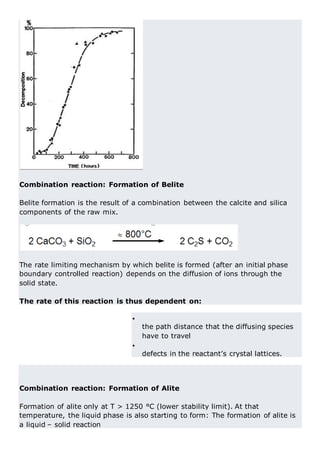
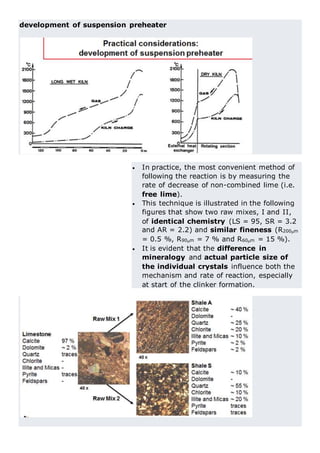
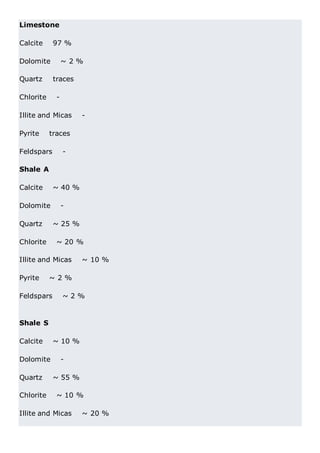
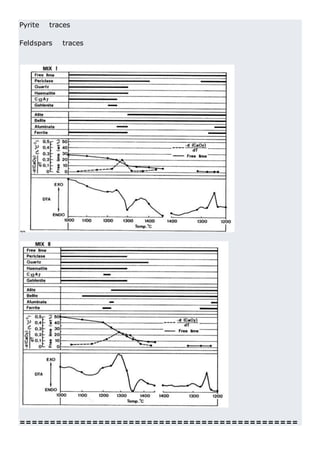
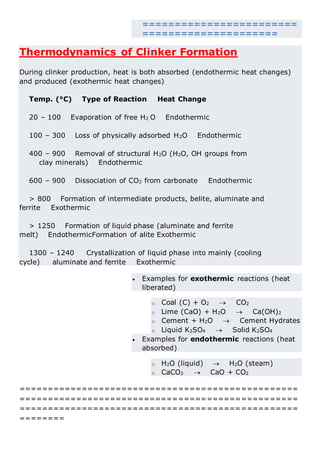
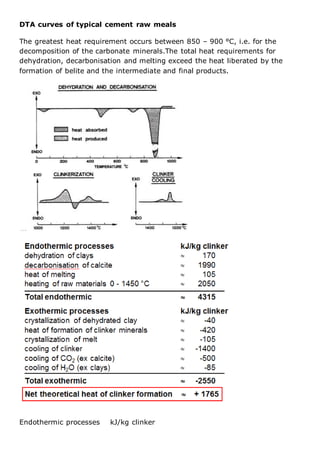
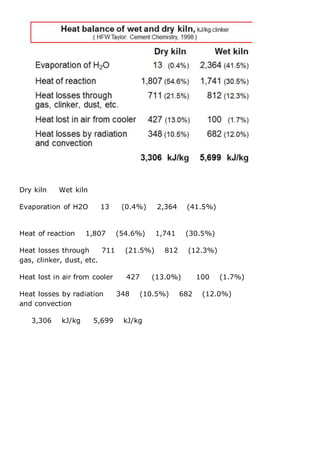
This document discusses clinker burning and the formation of Portland cement clinker. It begins with an introduction on why studying clinker burning is important. It then covers normal and abnormal kiln operation, and discusses the analogy to metamorphic rock formation. The document outlines the two principal steps of raw material disintegration and structural rearrangement during heating. It also discusses features of clinker formation like being a complex system that requires energy and produces minerals that are not stable at normal temperatures. The document provides details on reaction pathways, mechanisms, kinetics, thermodynamics, and intermediate products involved in clinker formation. It closes with sections on controlling the burning process and assessing raw meal burnability.