Embed presentation
Downloaded 21 times



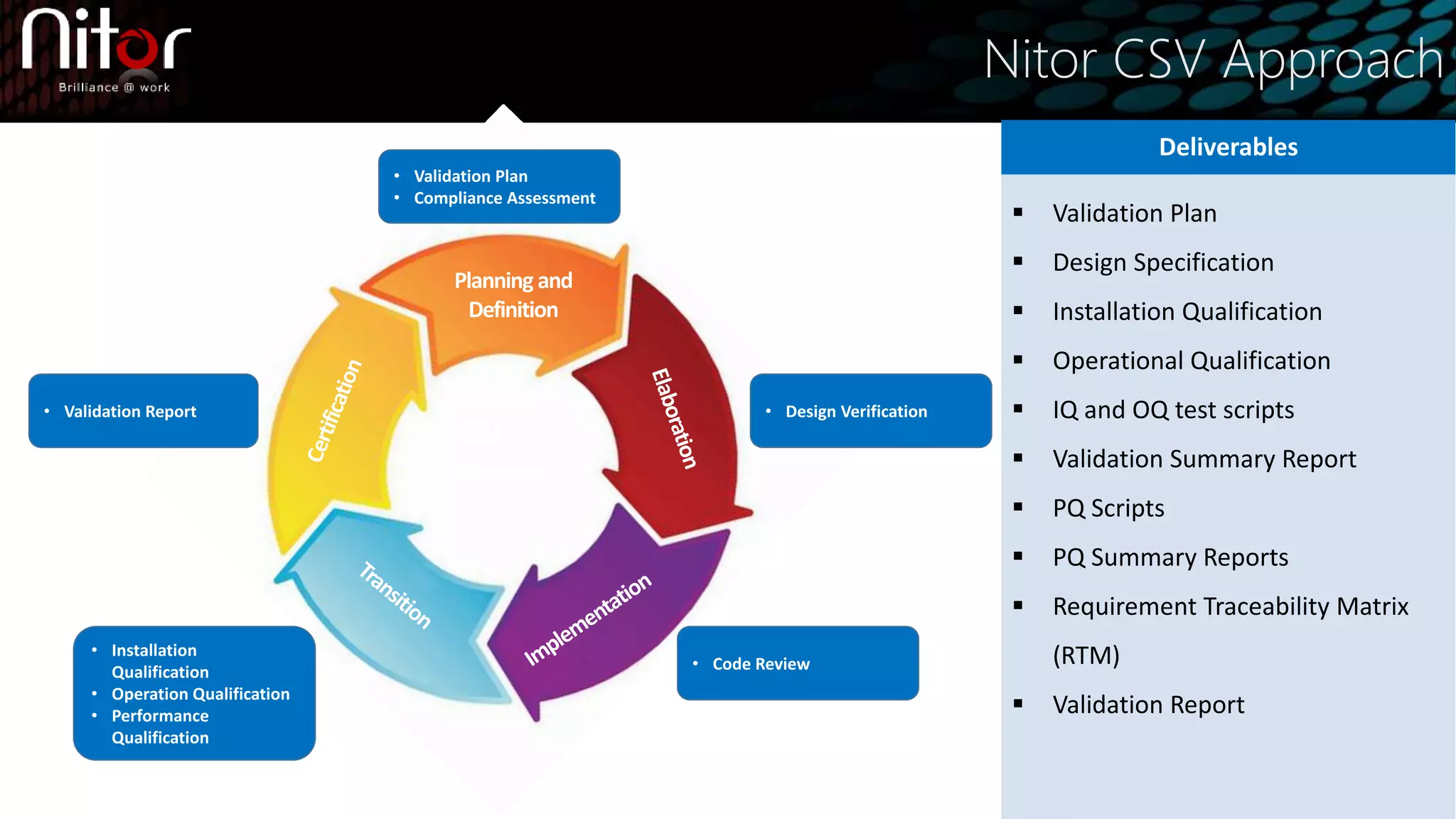
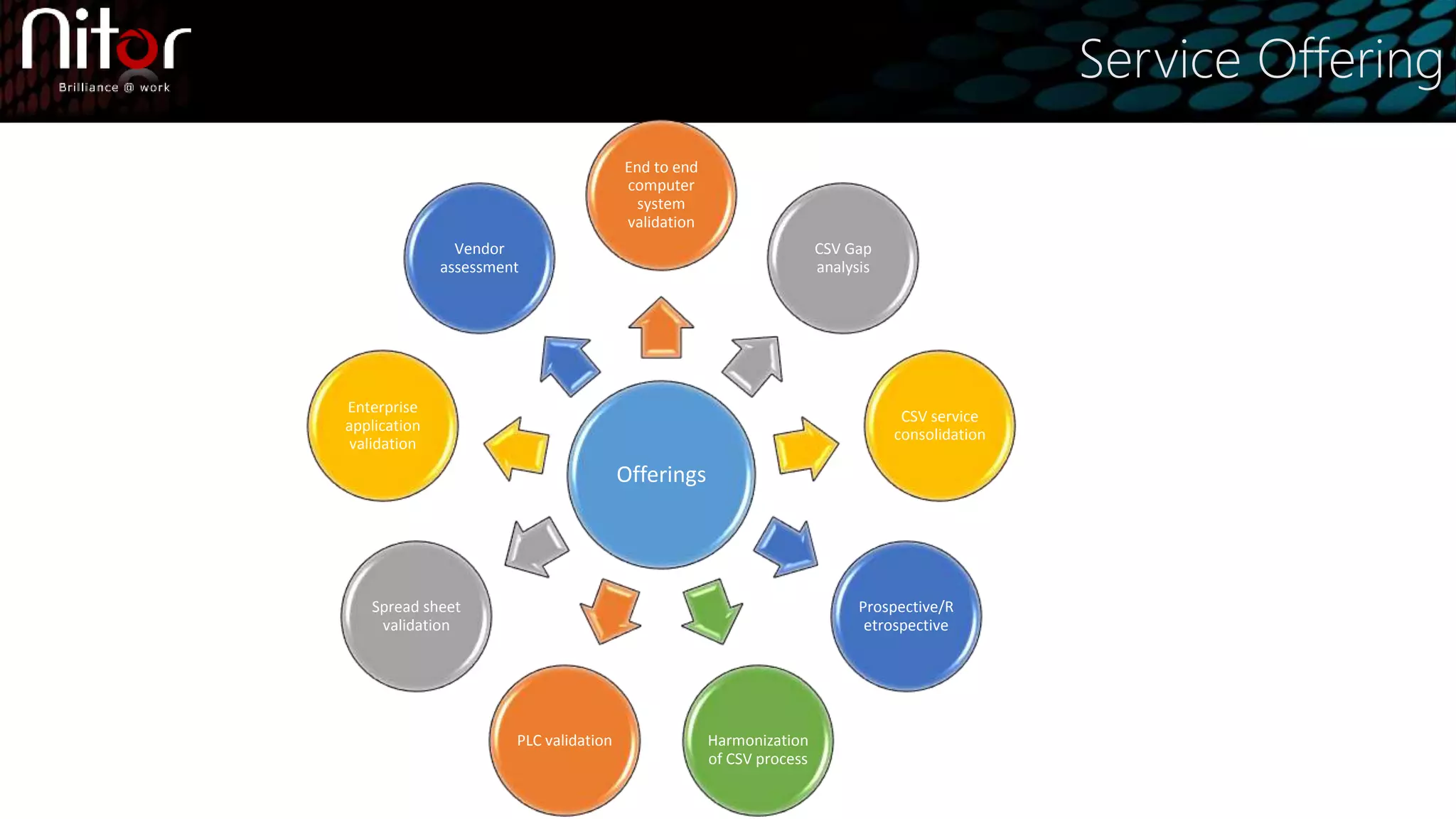



Nitor Infotech provides computer system validation (CSV) services to ensure regulatory compliance and prevent issues. Their CSV approach includes validation planning, design verification, installation qualification, operational qualification, performance qualification, and code reviews. They offer end-to-end CSV as well as gap analyses, service consolidations, and validation of spreadsheets, enterprise applications, and PLC systems. CSV reduces risks and costs by documenting requirements for regulators and ensuring quality, compliance, and patient safety.






