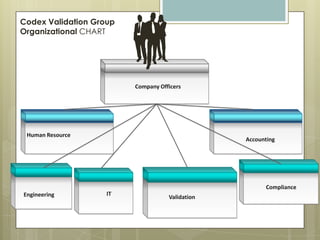
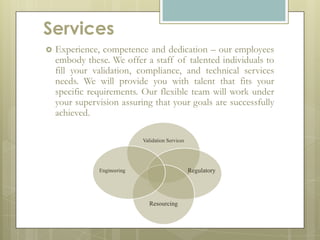
Codex Validation Group is an engineering staffing and validation services company founded in Puerto Rico by professionals with over 25 years of combined experience in the pharmaceutical industry. The company provides validation, engineering, IT, and regulatory compliance services with a focus on computer and automation system validation. Its team of experts have experience across the product development lifecycle from process engineering to packaging and equipment qualification. The company aims to become a leader in technical services and regulatory compliance solutions for manufacturing clients.