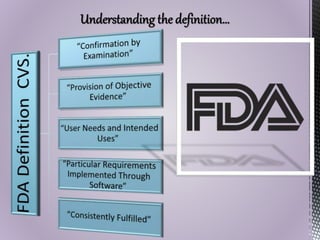
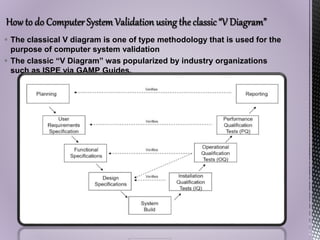
The document discusses computer system validation (CSV) in the pharmaceutical industry, outlining its definition, importance, and the requirements set by regulatory bodies such as the FDA. It explains the steps involved in CSV, including user needs assessment, design and functional specifications, testing phases, and documentation necessary for compliance. Furthermore, it highlights the consequences of noncompliance and the critical role of CSV in maintaining the integrity and reliability of systems used in drug production and distribution.