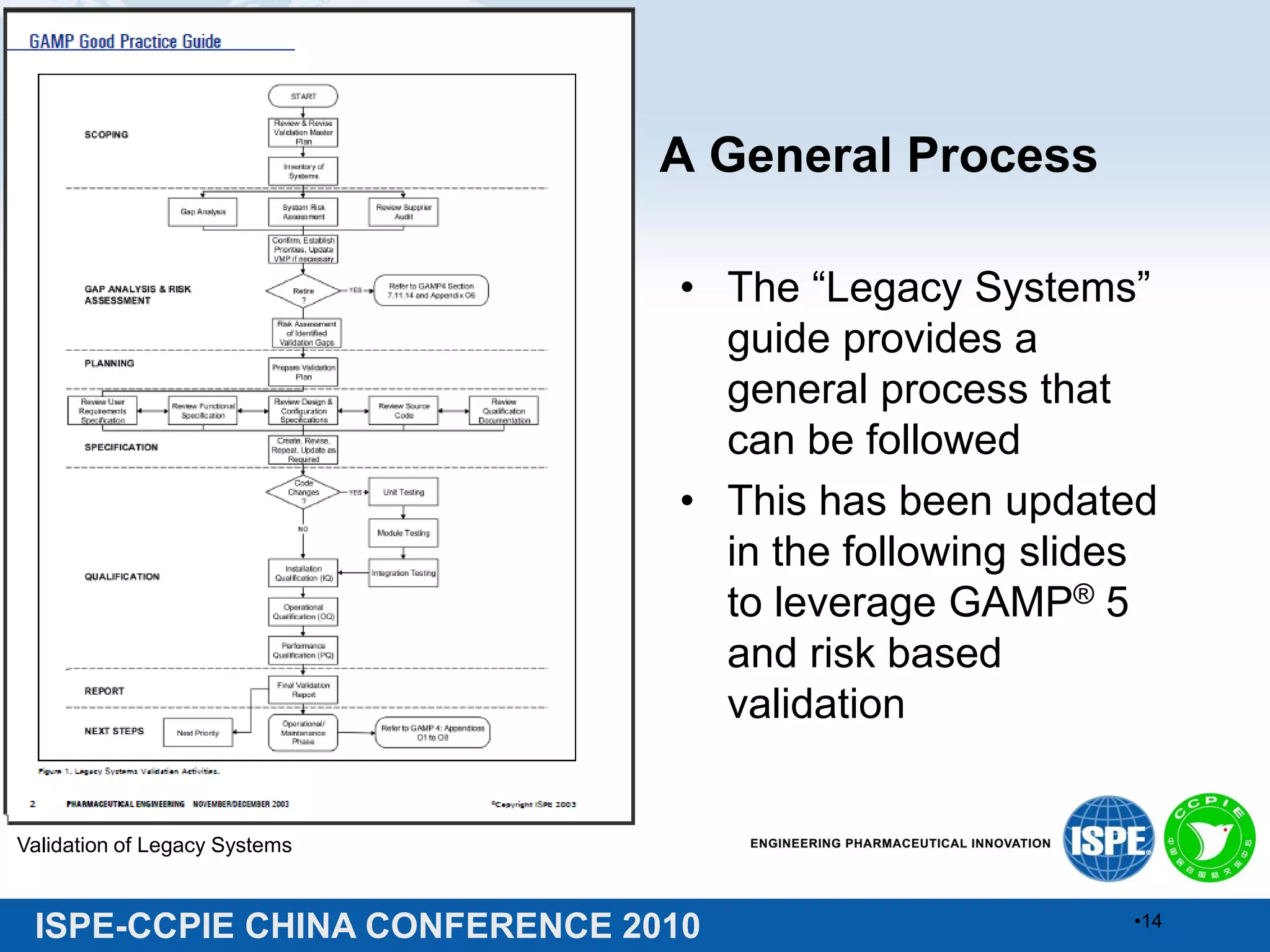
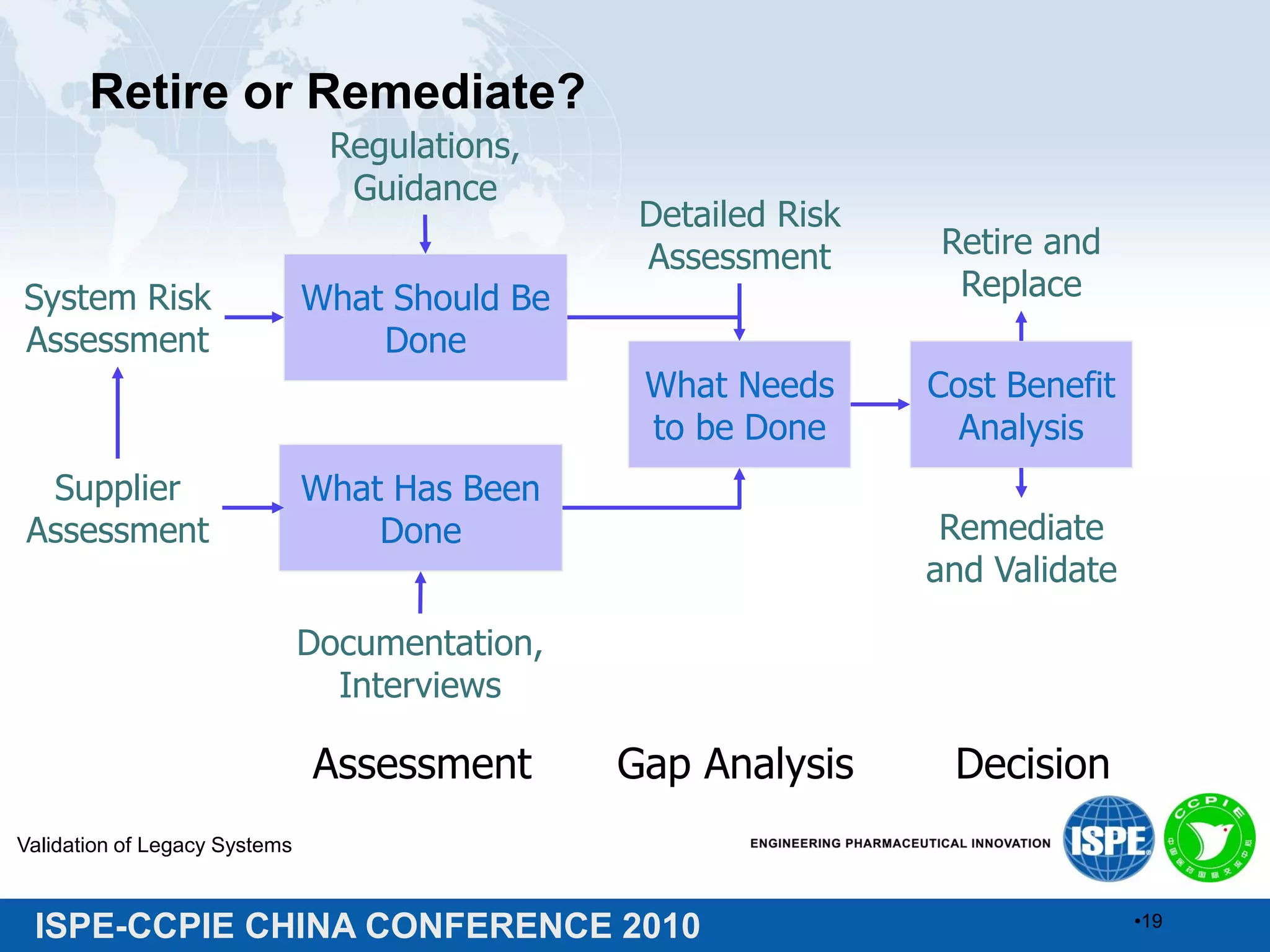
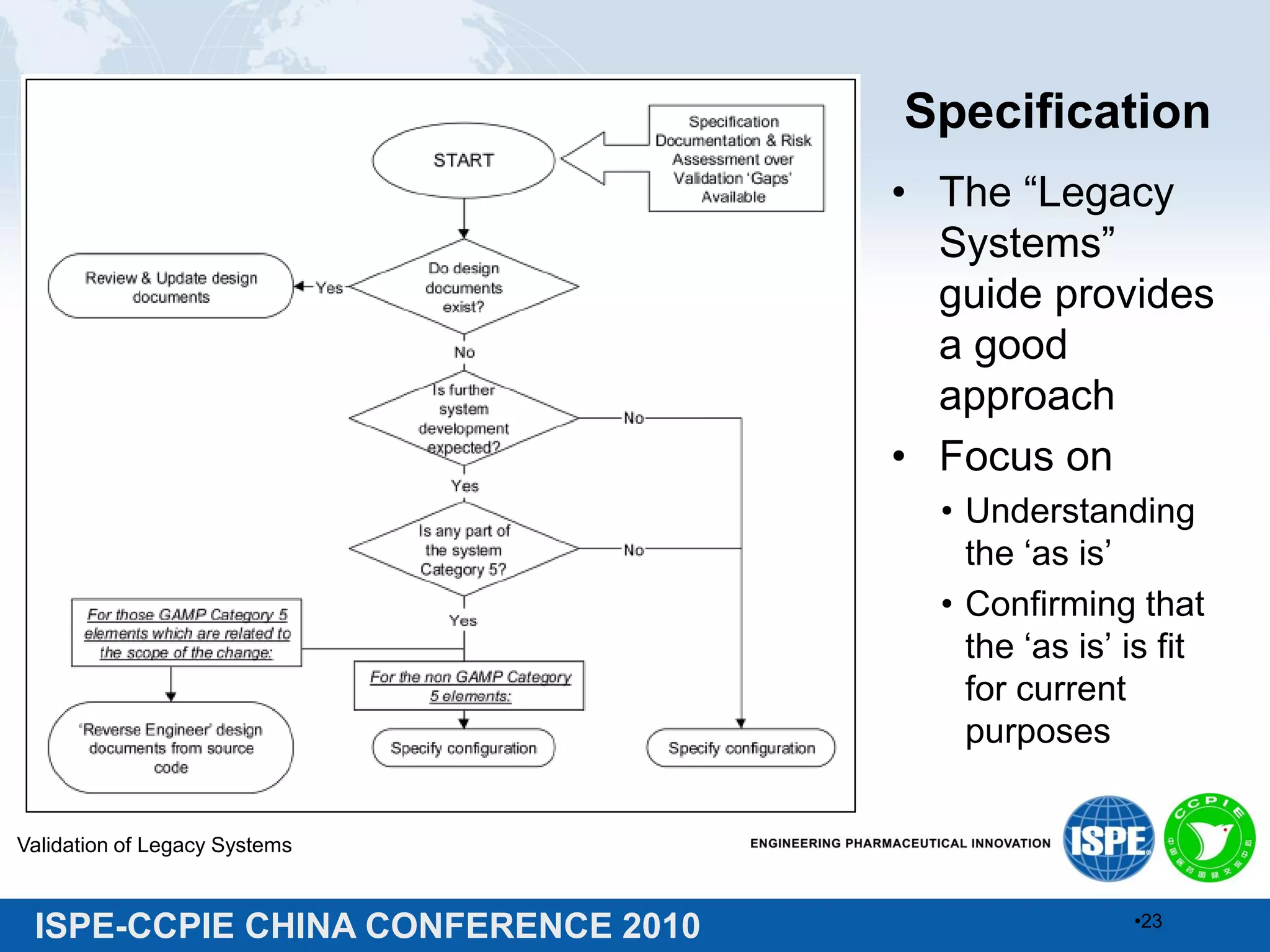
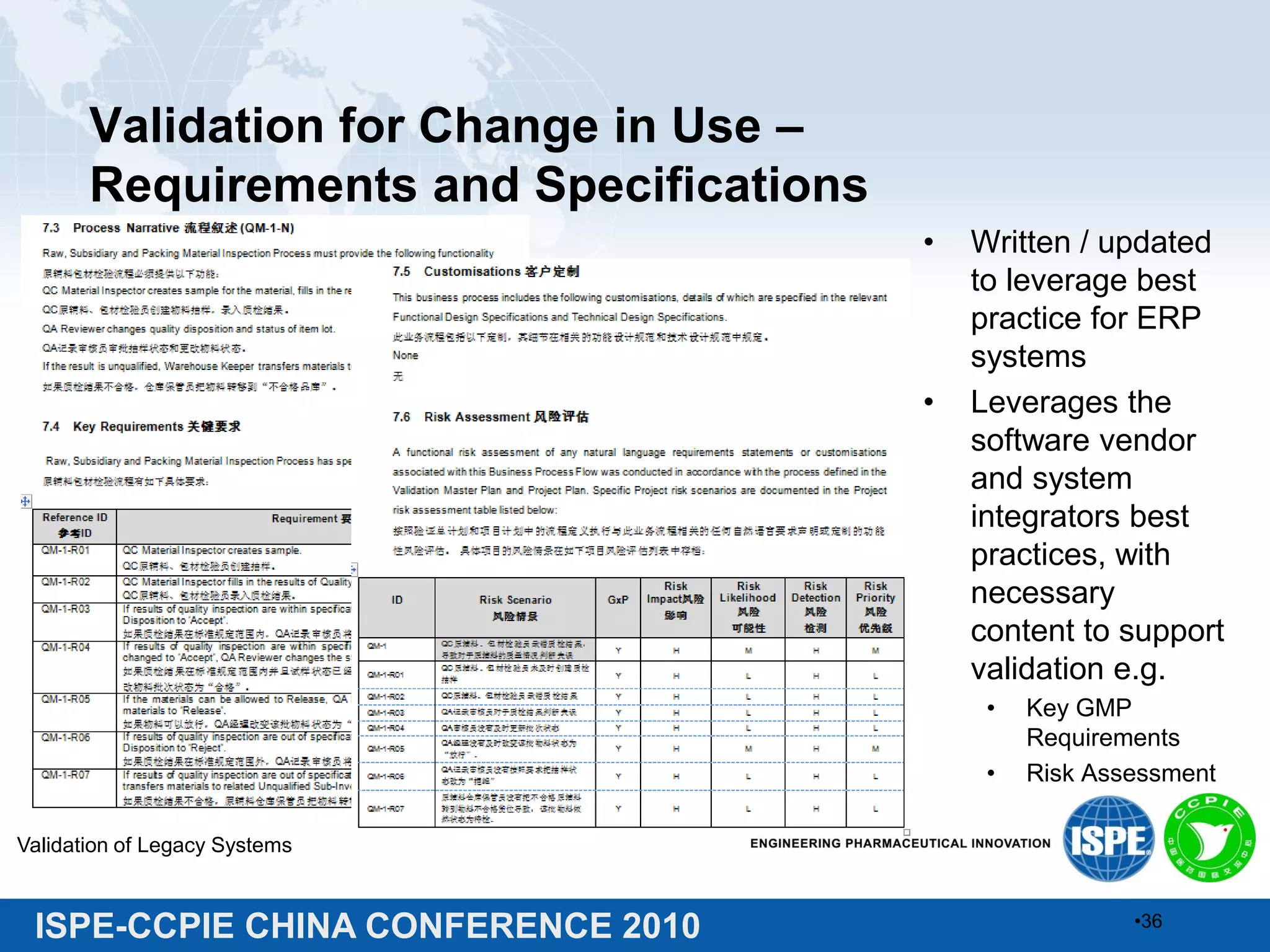
This document summarizes two presentations given at the ISPE-CCPIE China Conference 2010 on validating legacy systems. The first presentation discusses validating a global ERP system for a medical device company and a legacy ERP system for a Chinese pharmaceutical company. It provides guidance on assessing legacy systems, conducting a gap analysis, and developing a risk-based validation approach. The second presentation describes a retrospective validation of a global ERP system and a validation conducted for a change in use of a legacy ERP system in China.