The document discusses the significance of collagen, a connective tissue protein crucial for various body functions, including development, inflammation, and aging. It outlines the types, structure, biosynthesis, and disorders related to collagen, such as Ehlers-Danlos syndrome and osteogenesis imperfecta, which arise from genetic defects or nutritional deficiencies. Additionally, the document highlights the medical and industrial applications of collagen, including its use in cosmetic surgery, food products, and pharmaceuticals.

![The Extracellular Matrix
The space outside the cells of a tissue is filled with a composite material
called extracellular matrix(ECM).
This ECM is also known as the connective tissue. It is composed of -
a) Gel with interstitial fluid.
b) 3 major classes of biomolecules:
Structural proteins: collagen, elastin and Keratin(epidermal tissues)
Specialized proteins: e.g. fibrillin, fibronectin, and laminin.
Proteoglycans(Mucoproteins) : Conjugated proteins consisting of
Protein + Carbohydrate(5%-95%)
Carbohydrate part is in the form of Glycosaminoglycans [GAGs].
Thus, the extracellular matrix (ECM) is a complex structural entity
surrounding and supporting cells that are found within mammalian tissues.](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-3-320.jpg)





![Each alpha chain has an unusual
abundance of 3 amino-acids
glycine, proline and hydroxyproline.
Glycine occurs at every third
position in the amino acid sequence
which can be represented as (Gly-X-
Y)n.
X and Y are other amino acids of
which proline and hydroxyproline
occupy 100 positions each.
Glycine occupies the crowded
center of the triple helix as it has a
small side chain[ H atom] where as
Hydroxyproline and proline point
outwards imparting rigidity to the triple
helix.
The alpha chains are wound
around each other in a right handed
super helix to form a rod like molecule
1.4nm wide and 300nm long
[Gly-X-Y]n](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-9-320.jpg)
![The triple helices are stabilized by
Hydrogen bonds, covalent cross-
links, electrostatic and hydrophobic
interactions and van der waals forces.
The covalent cross links within and
between the helices are formed by
copper dependent enzyme lysyl
oxidase between the lysine and
hydroxylysine residues.
These triple helical molecules pack together
side by side to form elongated fibrils.
Fibrils are displaced longitudinally from each
other by 67 nm [one quatter of its length] to form
a quarter staggered arrangement.
Fibrils bundle up to form fibers making up
tissues.
Cross link formation](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-10-320.jpg)

![Biosynthesis of Collagen
Collagen synthesis occurs in the fibroblasts, osteoblasts in
bone, chondroblasts in cartilage and odontoblasts in teeth.
First synthesized in precursor form of preprocollagen polypeptide chain
in the ribosomes during translation
The leader sequence of amino acids[signal peptide] in the
preprocollagen directs it to enter the lumen of E.R
In the lumen of E.R, the Signal peptide is cleaved to form procollagen.
The proline and lysine amino acids in the procollagen chain undergo
hydroxylation and glycosylation known as post translational
modifications.
Disulfide bonds are formed between three procollagen chains which
twist around each other to form a triple helix molecule.This step is called
registration.
This Procollagen molecule is secreted into the extracellular matrix from
the golgi compartment of the E.R.
Here, the procollagen aminoproteinase and carboxy proteinase
enzymes remove extra terminal amino acids from the procollagen
molecule to form collagen .
The collagen molecules assemble into fibrils and inturn fibers being stabilized
by the covalent cross-links.](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-12-320.jpg)


![Abnormalities associated with collagen
Collagen-related diseases arise from
genetic defects
nutritional deficiencies
They affect the biosynthesis, assembly, postranslational modification, secretion, or
other processes involved in normal collagen production.
These include :
Ehler Danlos syndrome
Alport syndrome
Epidermolysis bullosa
Osteogenesis Imperfecta
Chondrodysplasias [affects cartilage]
Scurvy
Osteolathyrism](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-15-320.jpg)

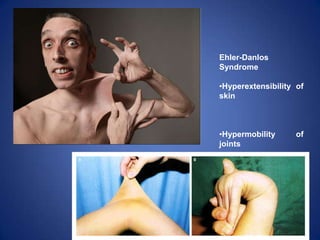
![Alport Syndrome
Alport syndrome is a genetic disorder
characterized by
glomerulonephritis, endstage kidney
disease, and hearing loss.
It also affects the eyes.
The presence of blood in the
urine[hematuria] is almost always found
in this condition.
CAUSE
Mutations in COL4A3,COL4A4,COL4A5
collagen biosynthesis genes.
These prevent the production or
assembly of the type IV collagen
network in the basement membranes.
kidneys are scarred and unable to filter
waste products resulting in hematuria
and renal disease.
Alport syndrome affecting eyes](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-18-320.jpg)



![Osteolathyrism
Osteolathyrism is a collagen cross-linking
deficiency caused by dietary over-
reliance on the seeds of Lathyrus sativus
(kesari dal) in some parts of India.
CAUSE
Osteolathyrogenic compounds like Beta-
aminopropionitrile(BAPN) and Beta-
oxalyl aminoalanine [BOAA] found in
Kesari dhal inhibit enzyme lysyl oxidase
required for the formation of cross links
in the triple helices
EFFECT
weakness and fragility of
skin, bones, and blood vessels
Paralysis of the lower extremities
associated with neurolathyrism](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-22-320.jpg)
![Scurvy
Scurvy is a disease due to deficiency of
vitamin C
It is not a genetic disease.
It is related to improper collagen
formation
CAUSE
Vitamin C [ascorbic acid ]is
required as a cofactor for
hydroxylase enzymes during the
hydroxylation of proline and lysine
in the synthesis of collagen.
Deficiency causes impaired
collagen synthesis due to
deficiency of hydroxylases.
EFFECT
Bleeding of gums
Poor wound healing
Subcutaneous hemorrhages](https://image.slidesharecdn.com/tissuefinal-130919110245-phpapp02/85/Collagen-The-connective-Tissue-Protein-23-320.jpg)