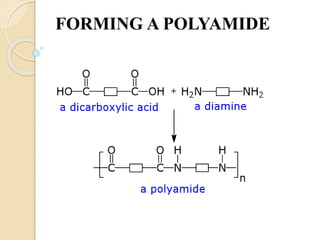
This document provides information on polymers, specifically polyamides and collagen. It discusses the structure, properties, formation, applications and history of polyamides, noting that they are macromolecules with repeating units linked by amide bonds. Examples include naturally-occurring proteins like wool and silk, and artificially-made nylons. Collagen is introduced as the most abundant protein in mammals, forming connective tissues. It has a triple helix structure and contains amino acids that give it strength. Both polyamides and collagen have various biomedical applications.