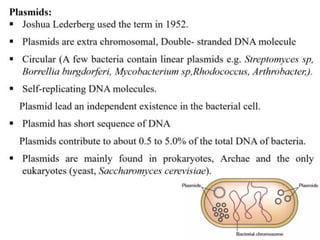
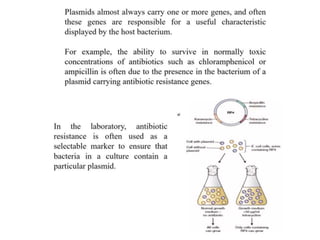
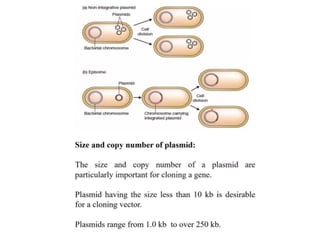
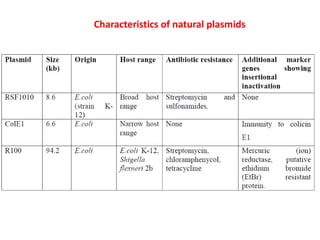
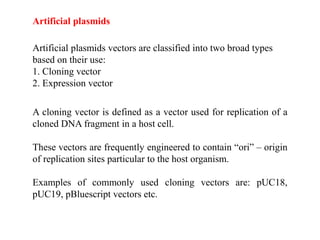
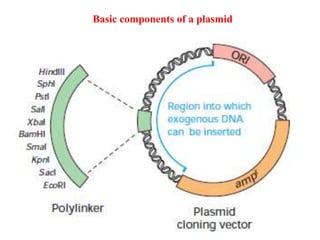
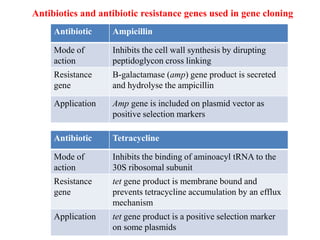
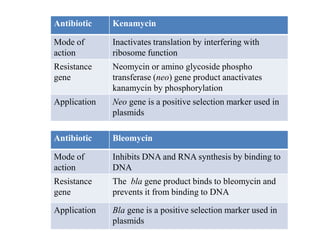
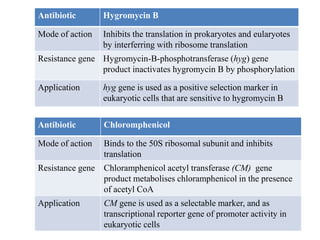
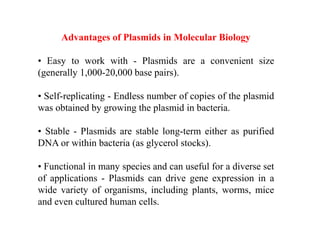
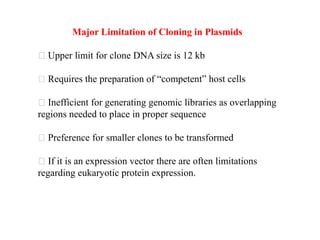
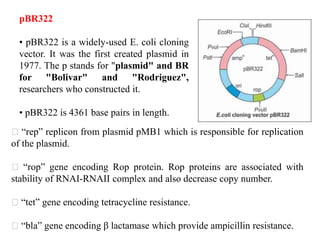
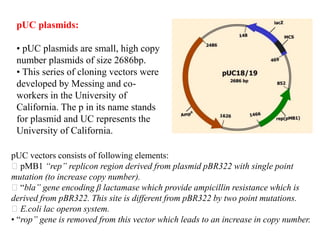
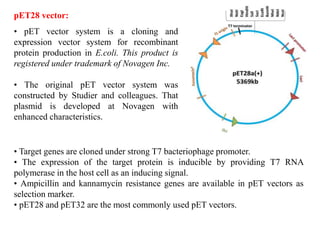
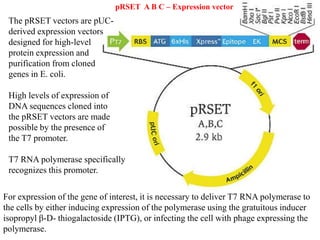
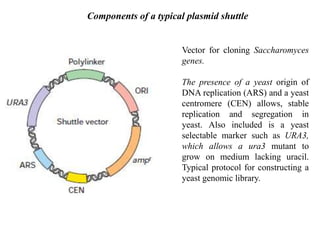
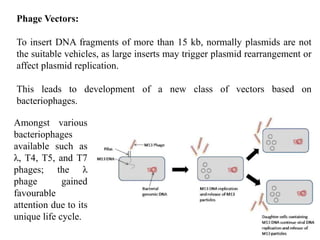
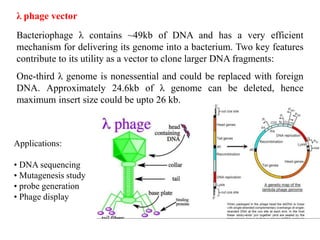
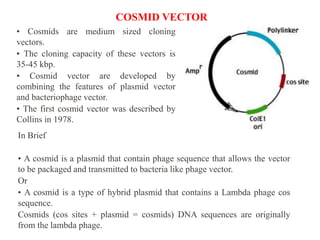
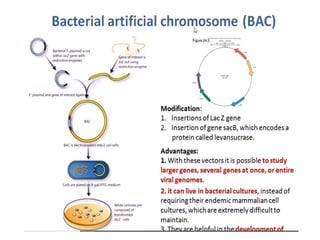
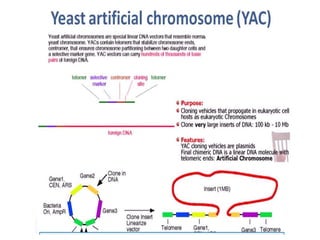
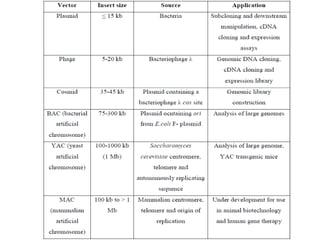
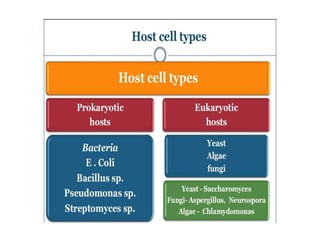
This document discusses different types of cloning vectors. It describes that vectors are used to carry foreign DNA into host cells. There are two main types of transformation vectors - cloning vectors which are used to increase copies of cloned DNA fragments, and expression vectors which are used to express foreign genes as proteins. Some examples of commonly used cloning vectors include pBR322 and pUC18/19, while examples of expression vectors include pET28 and pRSET vectors. The document then discusses various properties desirable in vectors such as an origin of replication, antibiotic resistance genes, and regulatory elements. It describes different types of vectors including plasmid vectors, bacteriophage vectors, cosmids, BACs/YACs, and mini chromosomes.