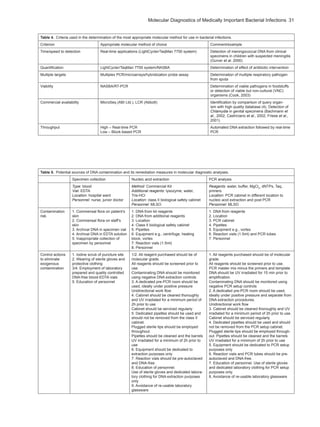
The document discusses the significance of molecular diagnostics in identifying medically important bacterial infections, emphasizing its role in enhancing patient care and managing infectious diseases. It outlines the evolution of molecular techniques, notably PCR, and their growing application in clinical microbiology over conventional methods. The chapter also examines historical developments in bacteriology and the essential need for reliable identification of pathogens to improve healthcare outcomes.