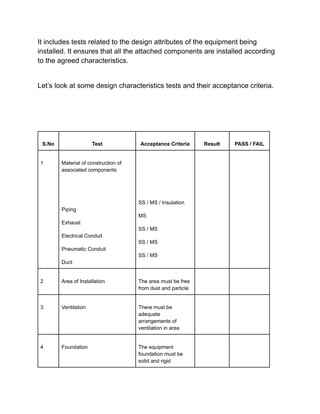
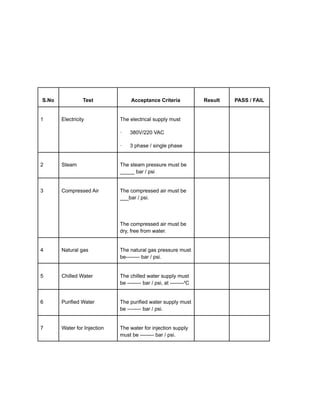
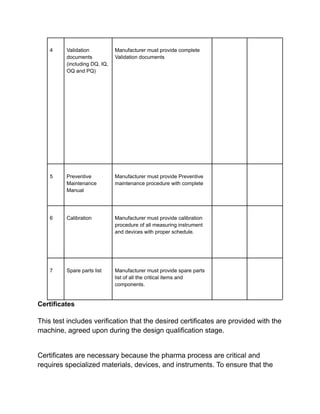
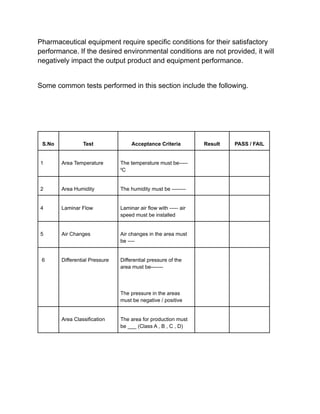
Installation qualification ensures that pharmaceutical equipment is correctly installed according to design requirements and manufacturer recommendations. It includes a series of tests documented in a format detailing test descriptions, acceptance criteria, and results to validate the installation. Documentation and certifications are crucial throughout the process to verify compliance and ensure proper operating conditions.