The document summarizes key concepts about matter including:
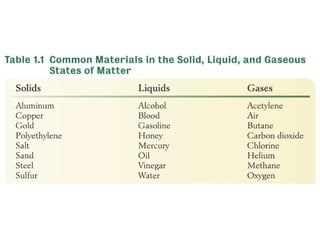
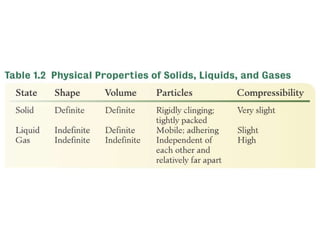
1. Matter can exist in different physical states such as solids, liquids, and gases which are determined by factors like the strength of attractive forces between particles and their ability to be compressed.
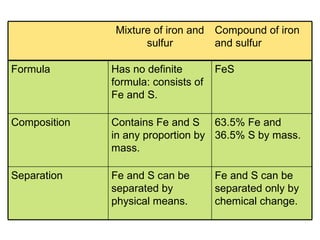
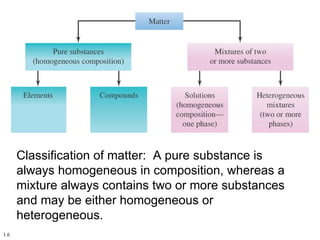
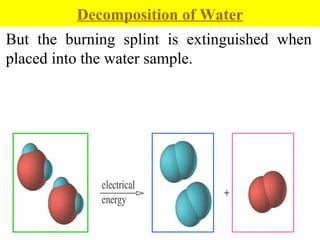
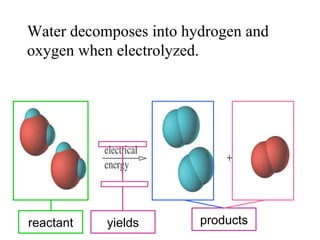
2. Pure substances have a uniform composition throughout while mixtures contain two or more substances and can be either homogeneous or heterogeneous.
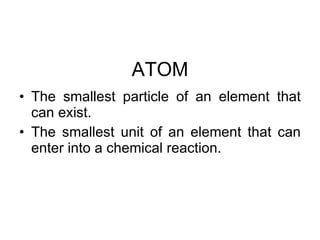
3. Elements are substances that cannot be broken down further while compounds contain two or more elements combined in fixed ratios.
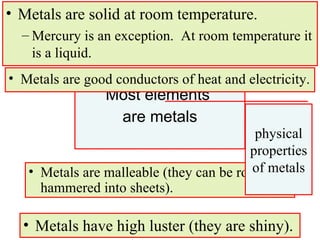
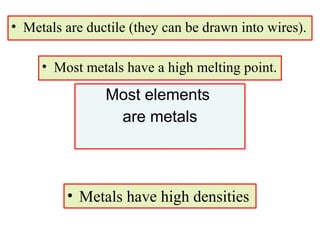
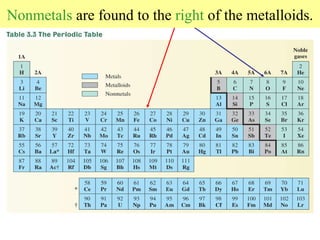
4. Metals, nonmetals, and metalloids have distinct physical and chemical properties depending on their position on the periodic table.