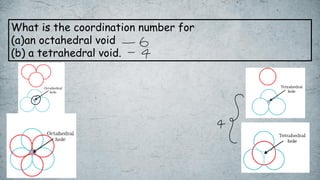
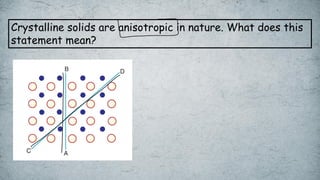
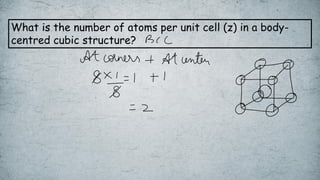
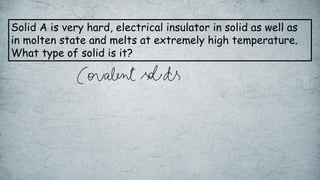
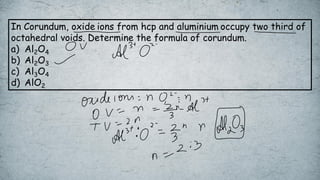
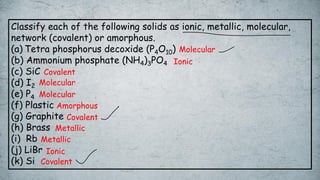
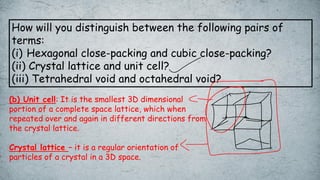
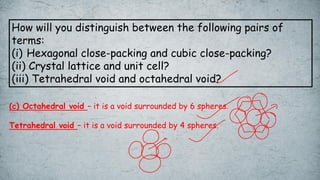
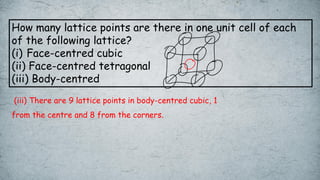
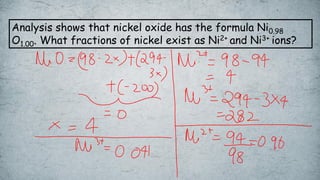
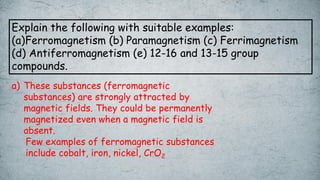
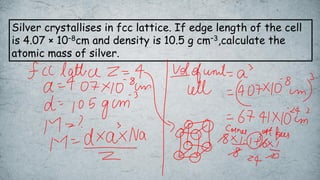The document discusses various concepts of solid-state chemistry, including types of magnetism, crystalline structures, and defects in solids. It includes questions and answers on topics like coordination numbers, types of solids, atomic mass determination, and examples of ferromagnetism and paramagnetism. Additionally, it covers topics such as the differences between tetrahedral and octahedral voids, types of close packing in crystal lattices, and calculations related to lattice points and atomic mass.