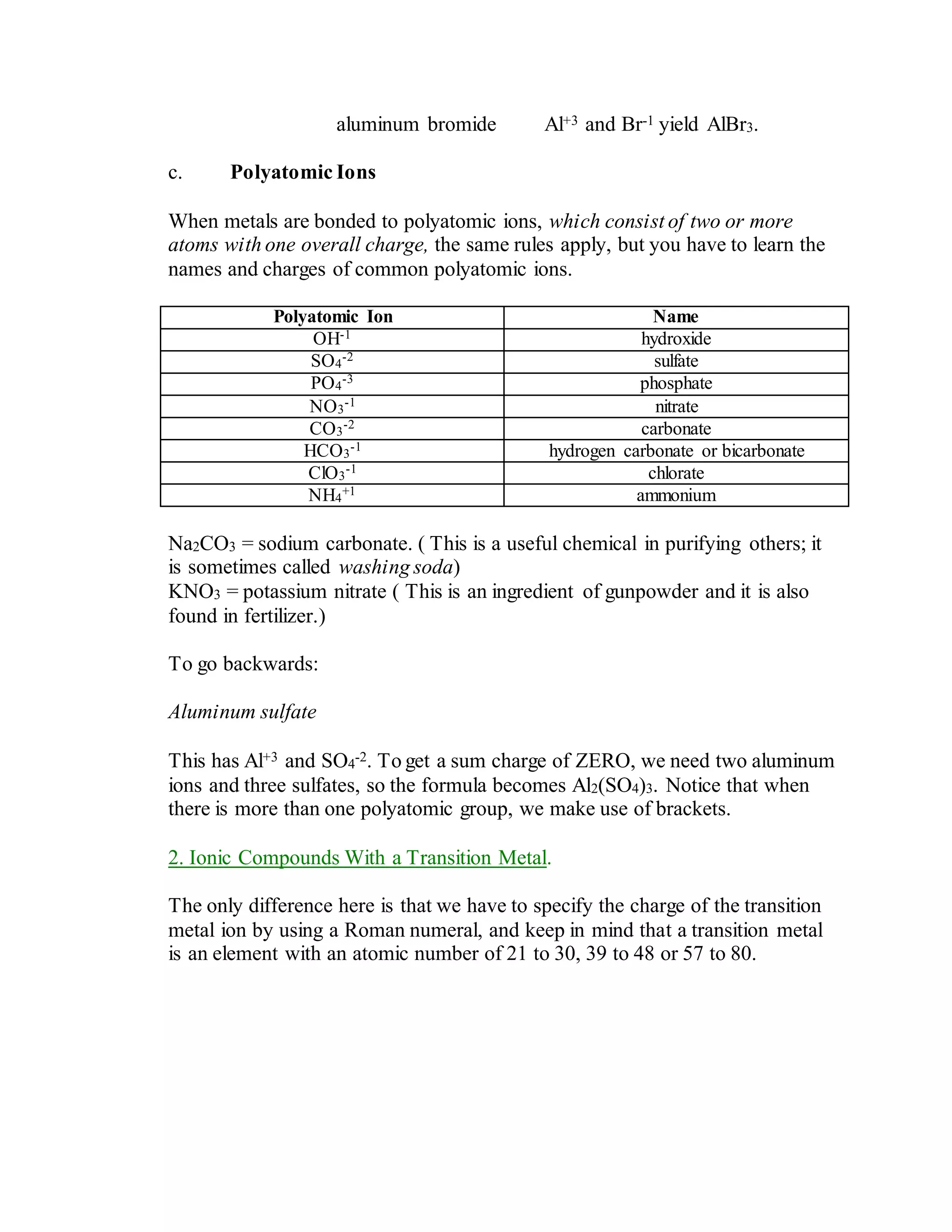
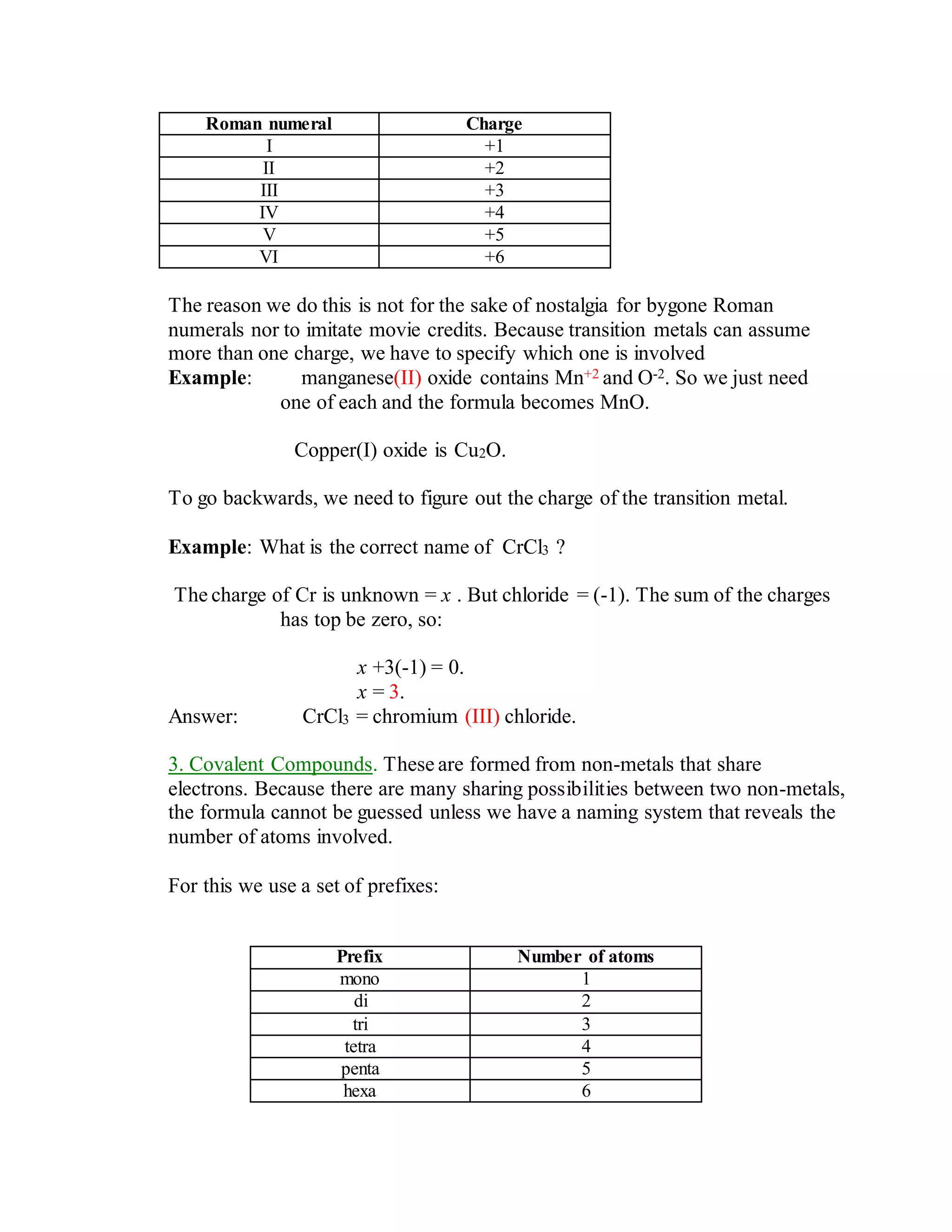
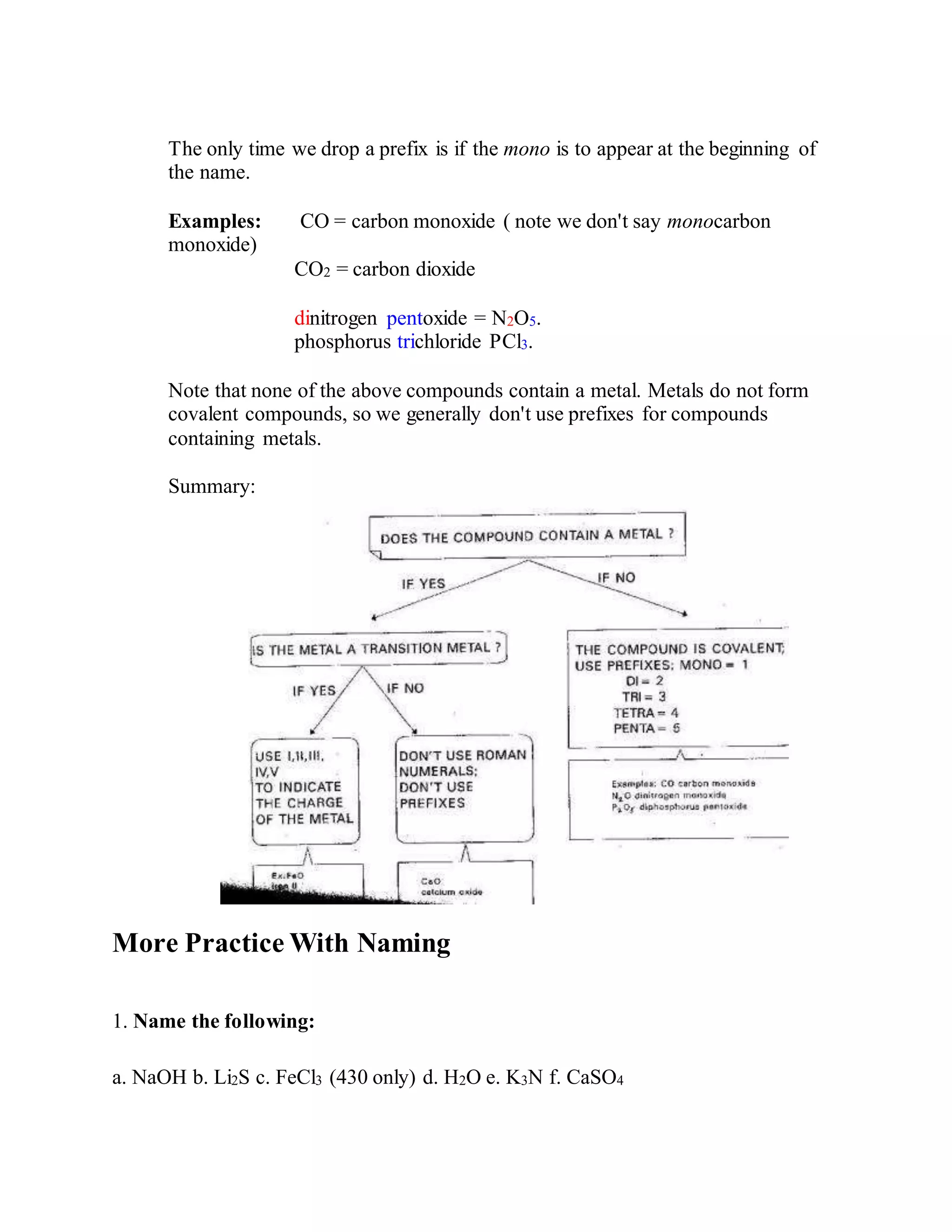
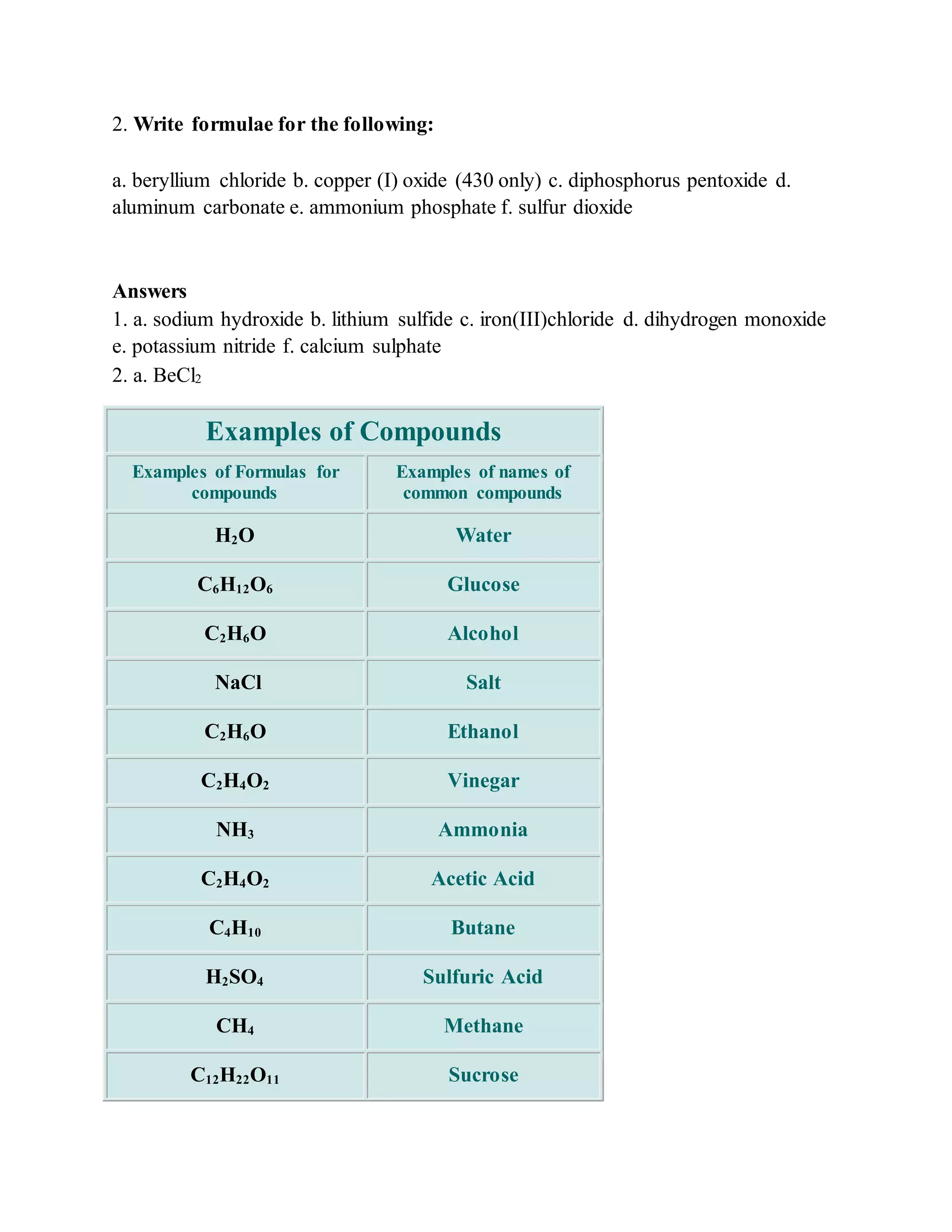
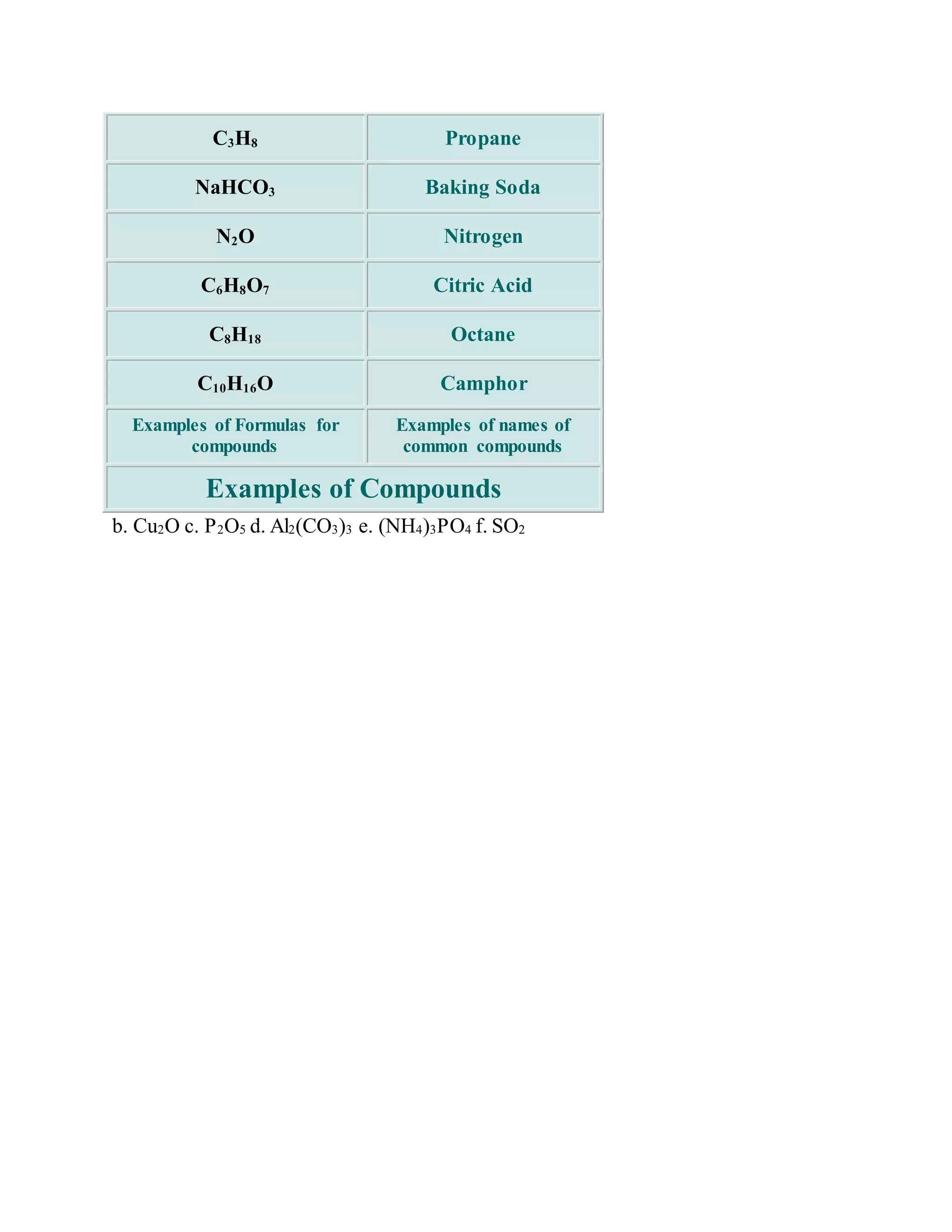
This document discusses the rules for naming ionic compounds, covalent compounds, and compounds containing transition metals. It provides examples of naming compounds from their formulas, such as sodium hydroxide (NaOH), lithium sulfide (Li2S), and iron(III) chloride (FeCl3). It also gives examples of writing formulas from compound names, like beryllium chloride (BeCl2), copper(I) oxide (Cu2O), and diphosphorus pentoxide (P2O5).