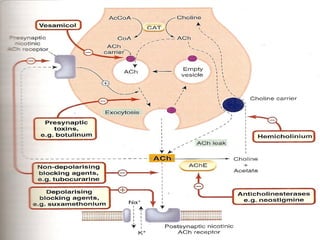
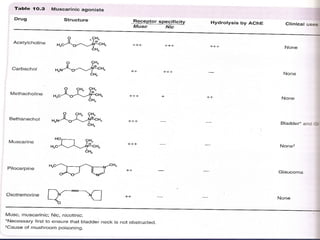
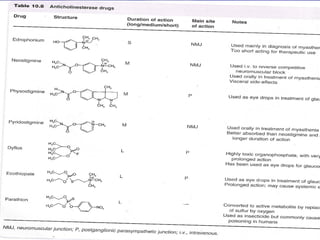
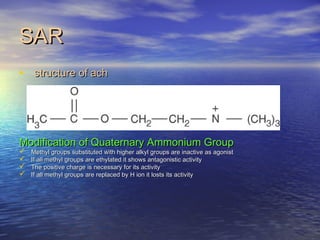
The seminar discusses cholinergic drugs, which affect the autonomic nervous system and are classified into muscarinic and nicotinic receptors based on their action. Key topics include the synthesis and release of acetylcholine, types of cholinergic agents, and their physiological effects, along with clinical applications in conditions such as glaucoma and myasthenia gravis. The document also covers the structure and modifications of these drugs, as well as references for further reading.












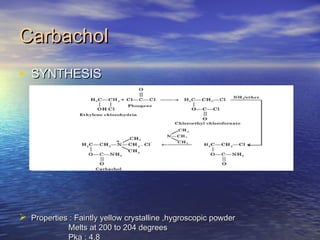
![CarbacholCarbachol
• StructureStructure
• Ethanaminium 2-[(aminocarbonyl) oxy]- N, N, N-Ethanaminium 2-[(aminocarbonyl) oxy]- N, N, N-
trimethyl-chloridetrimethyl-chloride](https://image.slidesharecdn.com/bharath007-101119062527-phpapp02/85/Cholinergic-drugs-ppt-17-320.jpg)