The document discusses acetylcholine (ACh) as a major neurotransmitter involved in various physiological functions, its biosynthesis, storage, release, and metabolism. It details the classes of cholinergic receptors (nicotinic and muscarinic), their distribution, and the pharmacological actions of various cholinergic drugs, including directly and indirectly acting agents. Additionally, it explores specific drugs such as carbachol, methacholine, and bethanechol, along with their therapeutic uses and mechanisms of action.






















![Physostigmine.
Physostigmine, a tertiary amine, is an alkaloid
obtained from the Calabar bean of West Africa
(Physostigma venenosum).
Chemically it is 1, 2, 3, 3α, 8, 8α -hexahydro-
1,3α,8-trimethyl-pyrrolo [2,3-b] indol-5-ol-methylcarbamate.
MOA.
Physostigmine reversibly inhibits acetycholinesterase thus results in
potentiation of cholinergic activity.
It stimulates muscarinic, nicotinic sites of ANS and nicotinic receptors of
neuromuscular junction.
Uses.Physostigmine has been used in the treatment of
1. glaucoma,
2. atropine intoxication and
3. overdose with the tricyclic antidepressants.](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-23-2048.jpg)






![O
O
S
P
O
S
O
O
Malathion is another effective pesticide, which is more effective on insects
than on humans because it requires biotransformation to the phosphate form,
which can only be carried out by insects. Malathion is a phosphodithioate ester.
Chemically it is 2-[(dimethoxyphosphinothioyl)thio]-butanedioic acid diethyl
ester. It is a poor irreversible inhibitor of choline esterase enzyme.
S
O
O
O
Malathion
diethyl 2-((dimethoxyphosphorothioyl)
thio)succinate
Uses.
Malathion is used extensively for controlling insects on vegetables, fruits,
and cereal crops.
It is also used for controlling insects affecting man and animals.](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-30-2048.jpg)







![I. Natural Alkaloids-
The natural alkaloids are found in plants of the solanaceae family. The levo-
isomers are much more active than the dextroisomers.
Atropine. It is anticholinergic that blocks muscarinic receptors. It was the first
anticholinergic alkaloid extracted from Solanaceae plant. It is an ester of tropine
and tropic acid and used as a sulphate salt in racemic form. At therapeutic does it
can penetrate the brain and stimulate the CNS
Uses
Uses
– Treat Bardycardia
– Reduce secretion before surgery
– Treat Iritis (painful inflammation of eye)
– Organophosphate poisoning
(only to decrease muscarinic action, not an
antidote like PAM)
MOA – It competitively binds to muscarinic receptor and antagonizes it thus
blocking all cholinergic effects
Atropine
8-methyl-8-azabicyclo[3.2.1]
octan-3-yl 3-hydroxy-2-phenylpropanoate
O
O
OH
N](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-38-2048.jpg)


![Homatropine
It is a synthetic alkaloid and is official as
homatropine hydrobromide or
homatropine methylbromide.
Uses :
1. Homatropine is mainly used as mydriatic and
2. is preferred over atropine due to its more rapid and short duration of action.
3. Homatropine is used to treat peptic ulcer and gastro-intestinal spasm
II. Semi-synthetic Derivatives-
N O OH
O
Homatropine
8-methyl-8-azabicyclo[3.2.1]octan-3-yl
-2-hydroxy-2-phenylacetate
3. Homatropine is used to treat peptic ulcer and gastro-intestinal spasm
Ipratropium is a quaternary ammonium
derivative of atropine that acts as an
anticholinergic agent.
Use. It is used to treat the symptoms
of chronic obstructive pulmonary
disease and asthama.](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-41-2048.jpg)

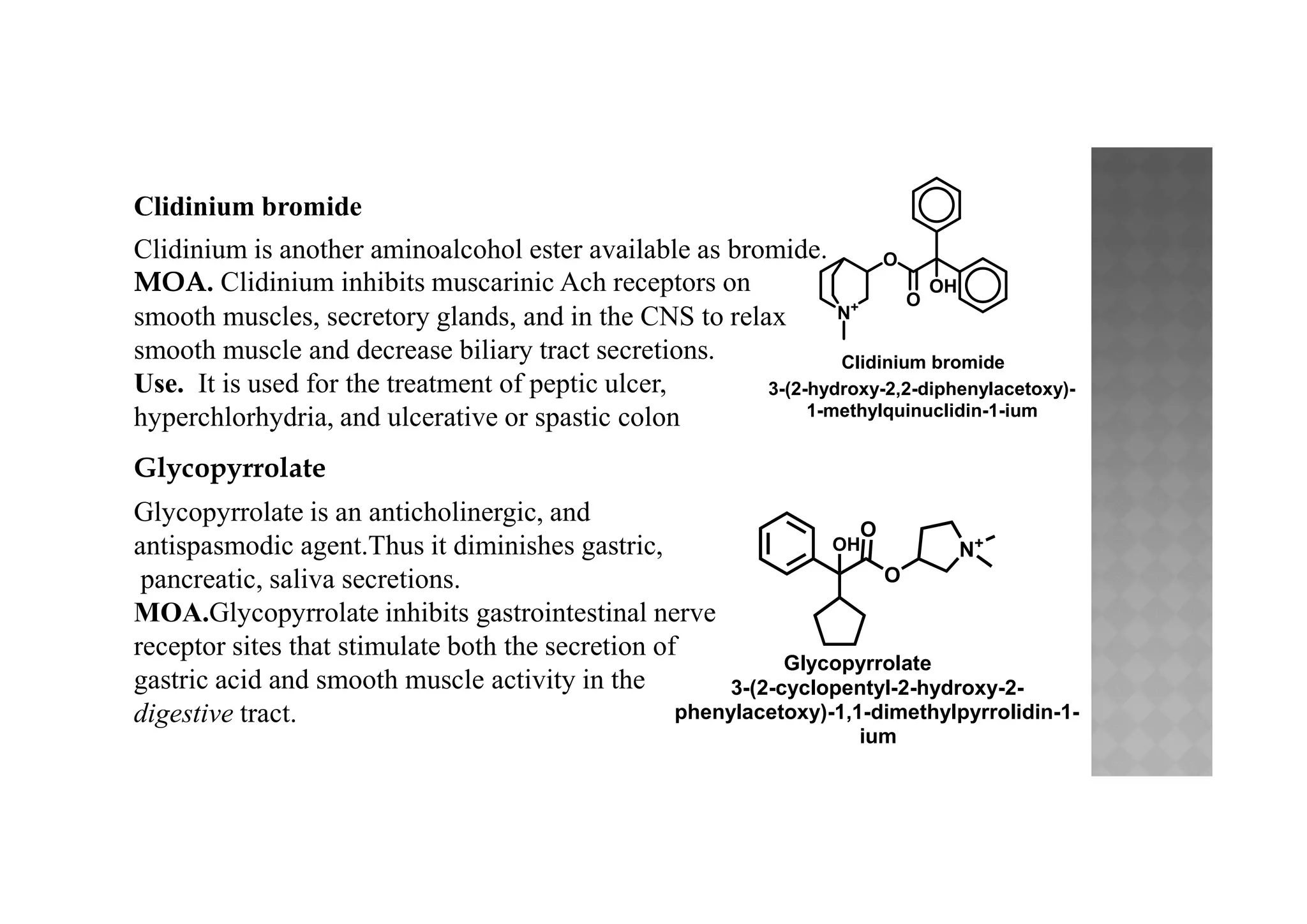
![Dicyclomine hydrochloride*
Dicyclomine is an aminoalcohol ester and is available
as hydrochloride salt.
Dicyclomine is synthesized from cyclohexylnitrile by the
following steps :
(a) Cyclohexylnitrile on alkylation with 1, 5-dibromopentane
yields a cyclohexane intermediate, which is hydrolysed to get acid.
Dicyclomine
O
O
N
2-(diethylamino)ethyl
[1,1'-bi(cyclohexane)]-1-carboxylate
(b) The above acid is esterified with N, N-diethylamino ethanol to get dicyclomine.](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-44-2048.jpg)

![Propantheline bromide is an antimuscarinic agent.
MOA. Propantheline is one of a group
of antispasmodic medications which work by
blocking the action of the chemical
messenger acetylcholine.
Use. It is used as antispasmodic and
in the treatment of peptic ulcers.
Benztropine mesylate
Benztropine mesylate
Benzatropine is a centrally acting anticholinergic
/antihistamine agent. It is a selective M1 muscarinic Ach
receptor antagonist.
Use. It is a medication used to treat a type of movement
disorder due to antipsychotics known as dystonia .
It is also a second-line drug for the treatment of
Parkinson's disease. It improves tremor, and may
alleviate rigidity and bradykinesia.
N O
Benztropine
3-(benzhydryloxy)-8-methyl-8-
azabicyclo[3.2.1]octane](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-46-2048.jpg)
![Orphenadrine citrate
Orphenadrine is an aminoalcohol ether derivative.
It has anticholinergic and antihistaminic activities.
It is closely related to diphenhydramine.
Uses. Orphenadrine is used for symptomatic treatment of
Parkinson’s disease. It is also used as skeletal muscle
relaxant.
O
N
N,N-dimethyl-2-(phenyl(o-
tolyl)methoxy)ethan-1-amine
Orphenadrine
Biperidine hydrochloride
Biperiden is a weak visceral anticholinergic agent.
It has strong nicotinolytic action.
Uses.
1. Biperiden is used to treat parkinson’s disease
2. It is also used to cure akineasia, rigidity and
tremor
Biperidine
OH
N
1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-
phenyl-3-(piperidin-1-yl)propan-1-ol](https://image.slidesharecdn.com/cholinergicsandanti-cholinergicdrugs-210505143149/75/Cholinergics-and-anti-cholinergic-drugs-47-2048.jpg)


