This document provides an overview of the topics that will be covered in the Biochemistry course Chem. 433H. The course will focus on biochemical aspects of enzymes, digestion, metabolism of carbohydrates, lipids and proteins, vitamins, and hormones. Specific topics that will be covered include enzymatic kinetics and regulation, digestive processes, glycolysis and the TCA cycle, beta oxidation of fatty acids, amino acid metabolism and the urea cycle, and the structure and function of insulin, thyroxine, and glucocorticoids. Readings from three biochemistry textbooks are suggested.


![Enzymes
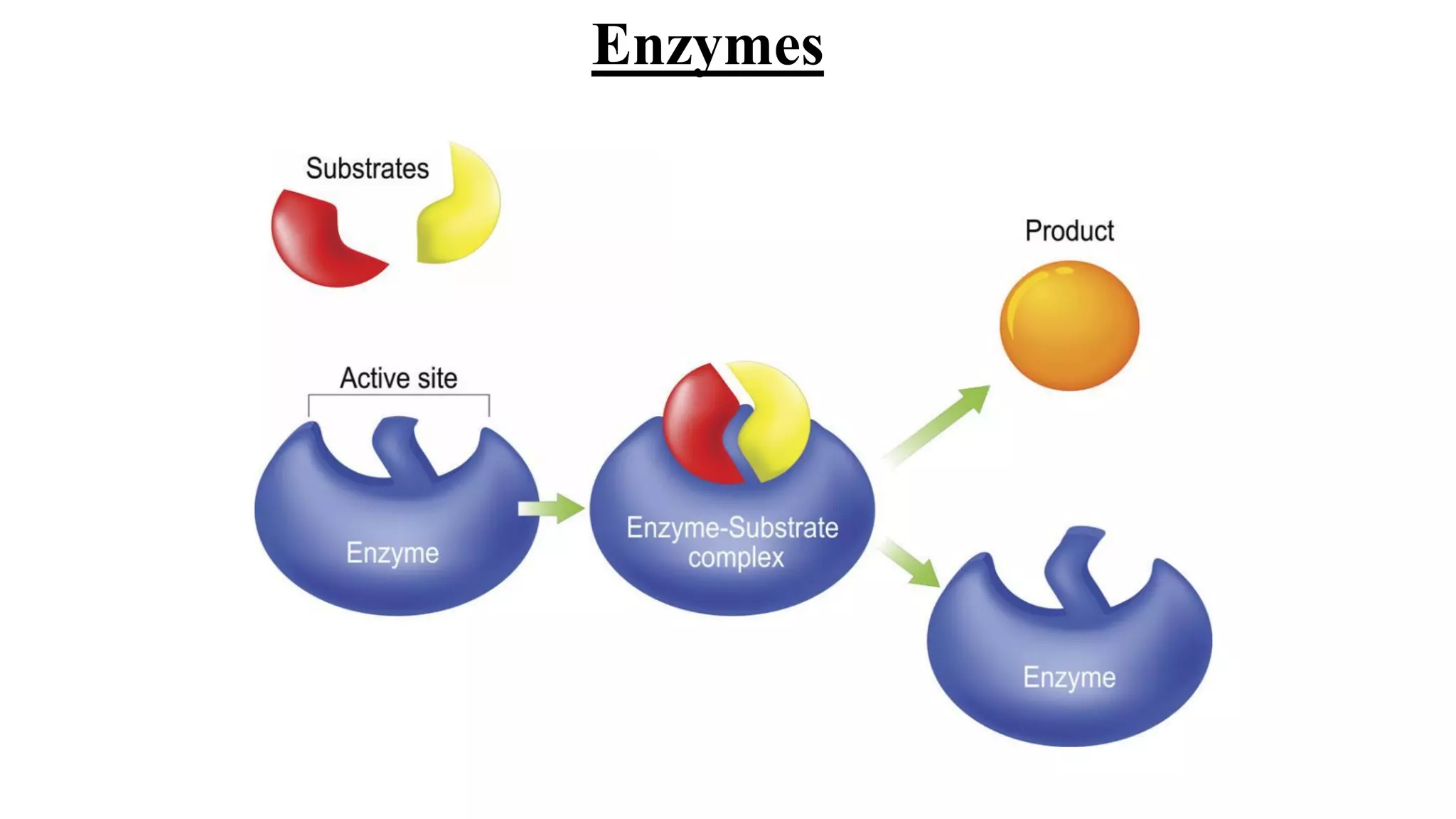
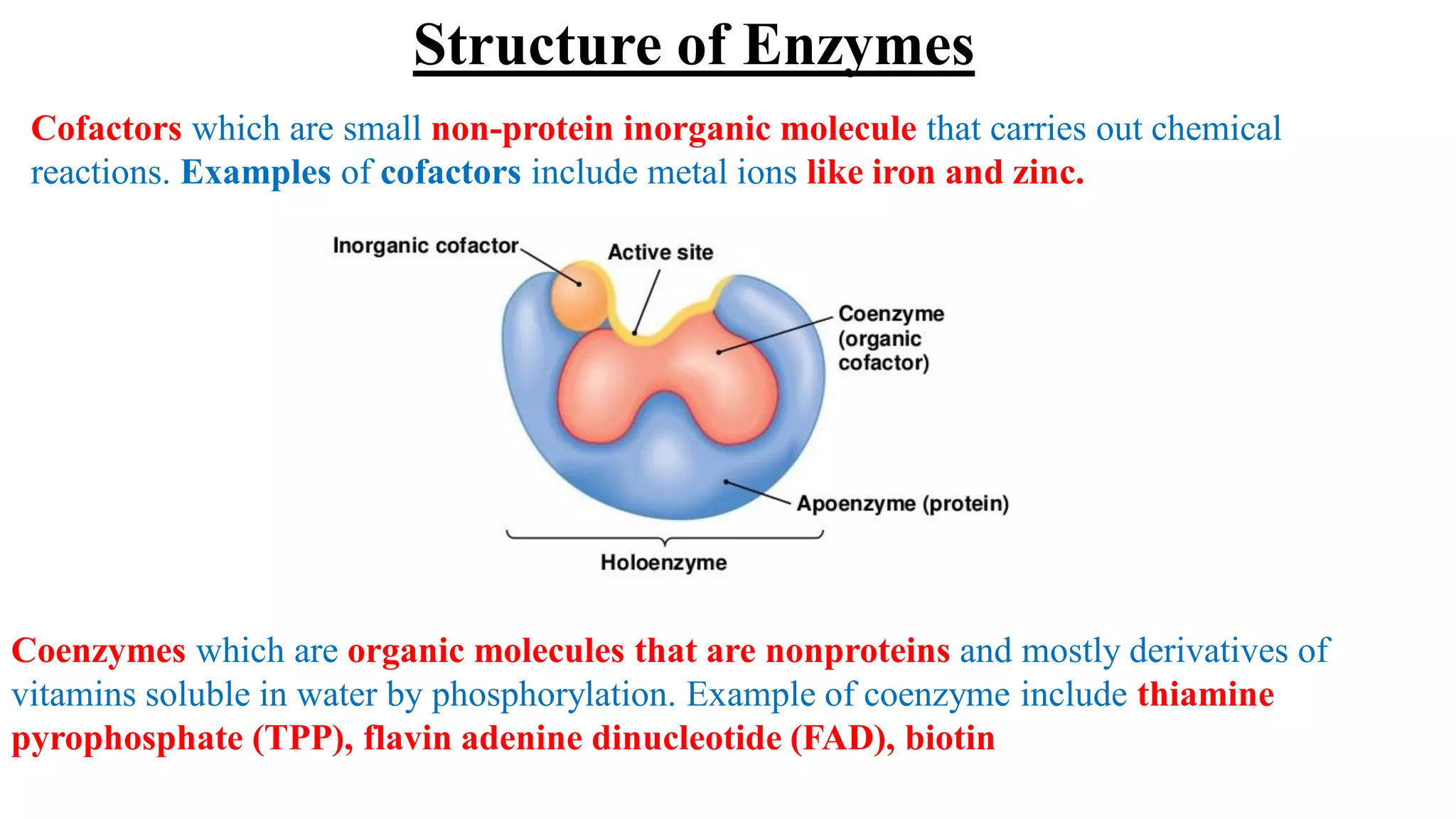
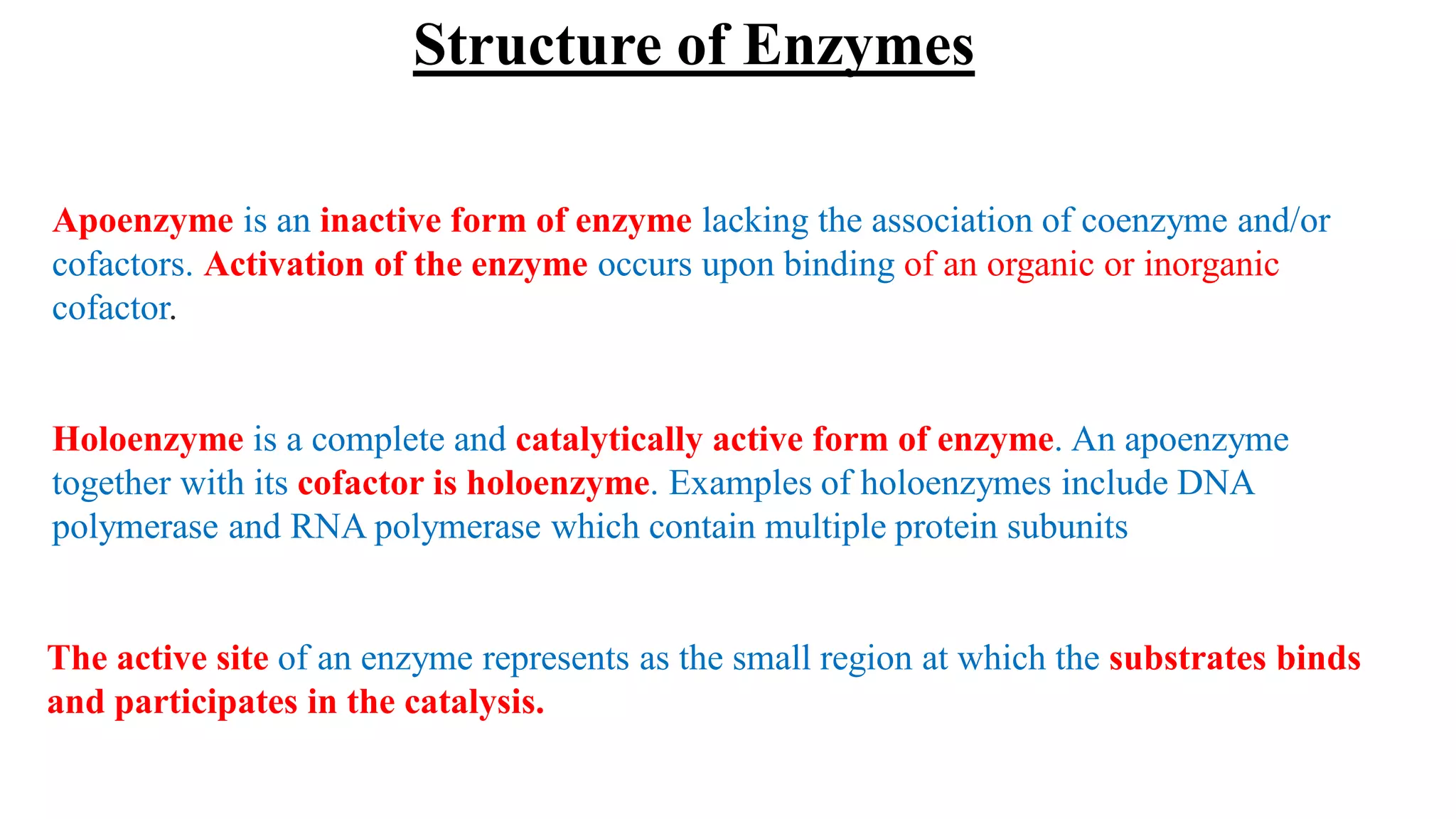
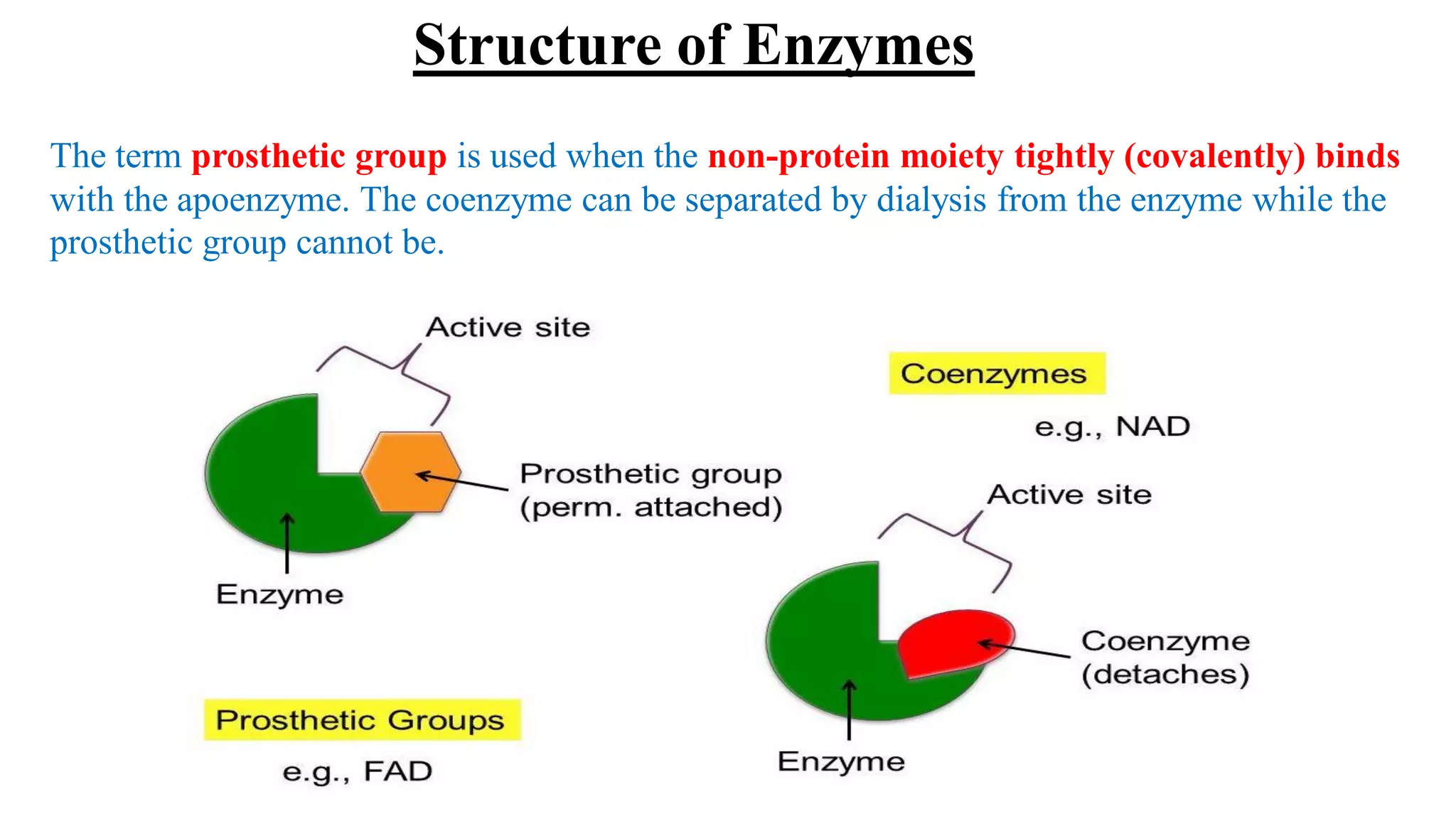
Enzymes are macromolecular biological catalysts that accelerate biochemical reactions.
They are highly specialized protein[exception-RNA acting as ribozyme] having
extraordinary catalytic power(greater than any organic or inorganic catalyst) and high
degree of specificity for their substrate.
Enzyme MW= 12000 to 1 million
Protein Amino acids
Amino acids
2-3 hrs, Enzyme HCl,37C
100 ̊C,Strong acid
2-3 days](https://image.slidesharecdn.com/lectureno-230818154250-ff047983/75/Lecture-No-1-01_02_21-pdf-4-2048.jpg)