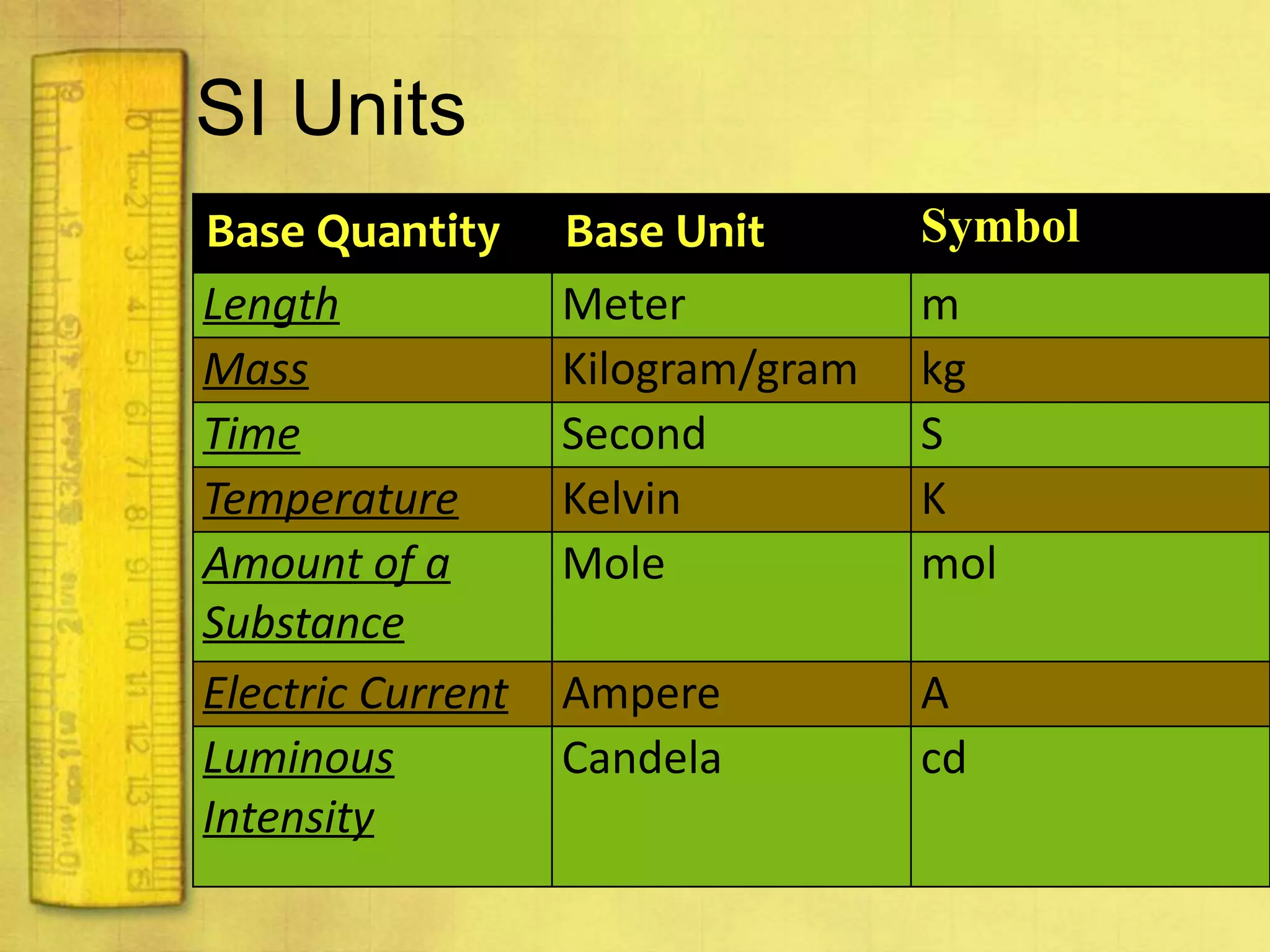
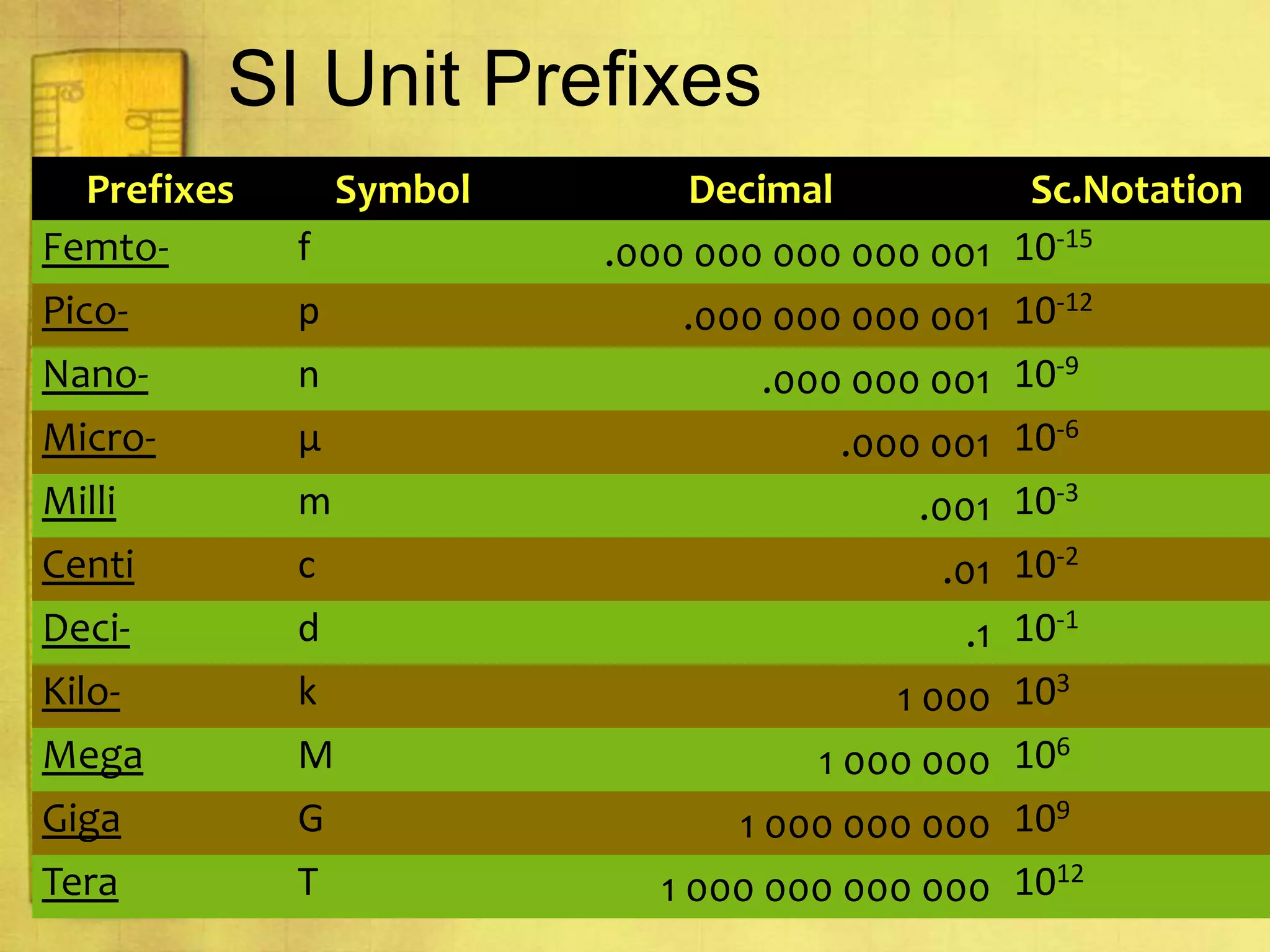
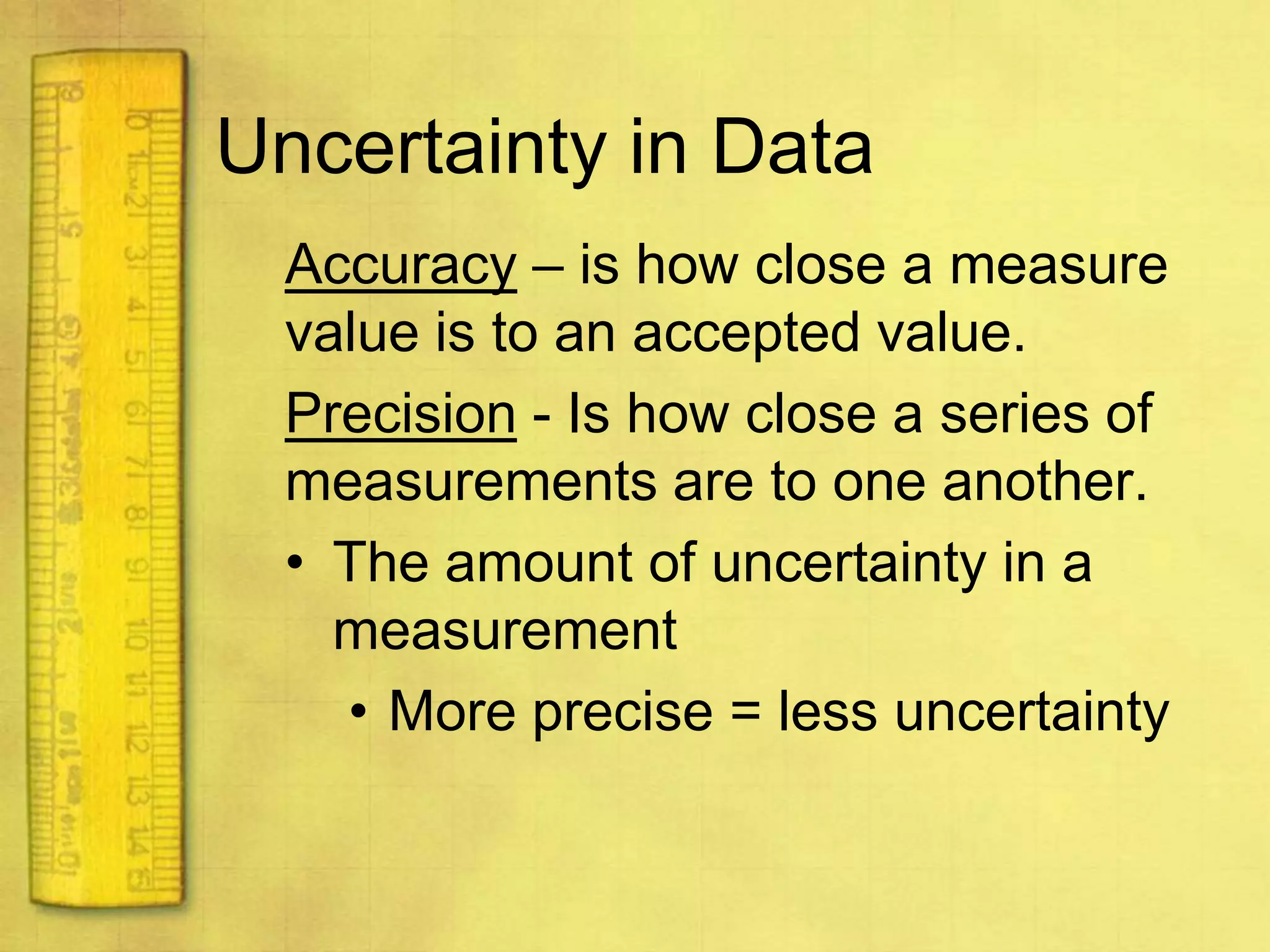
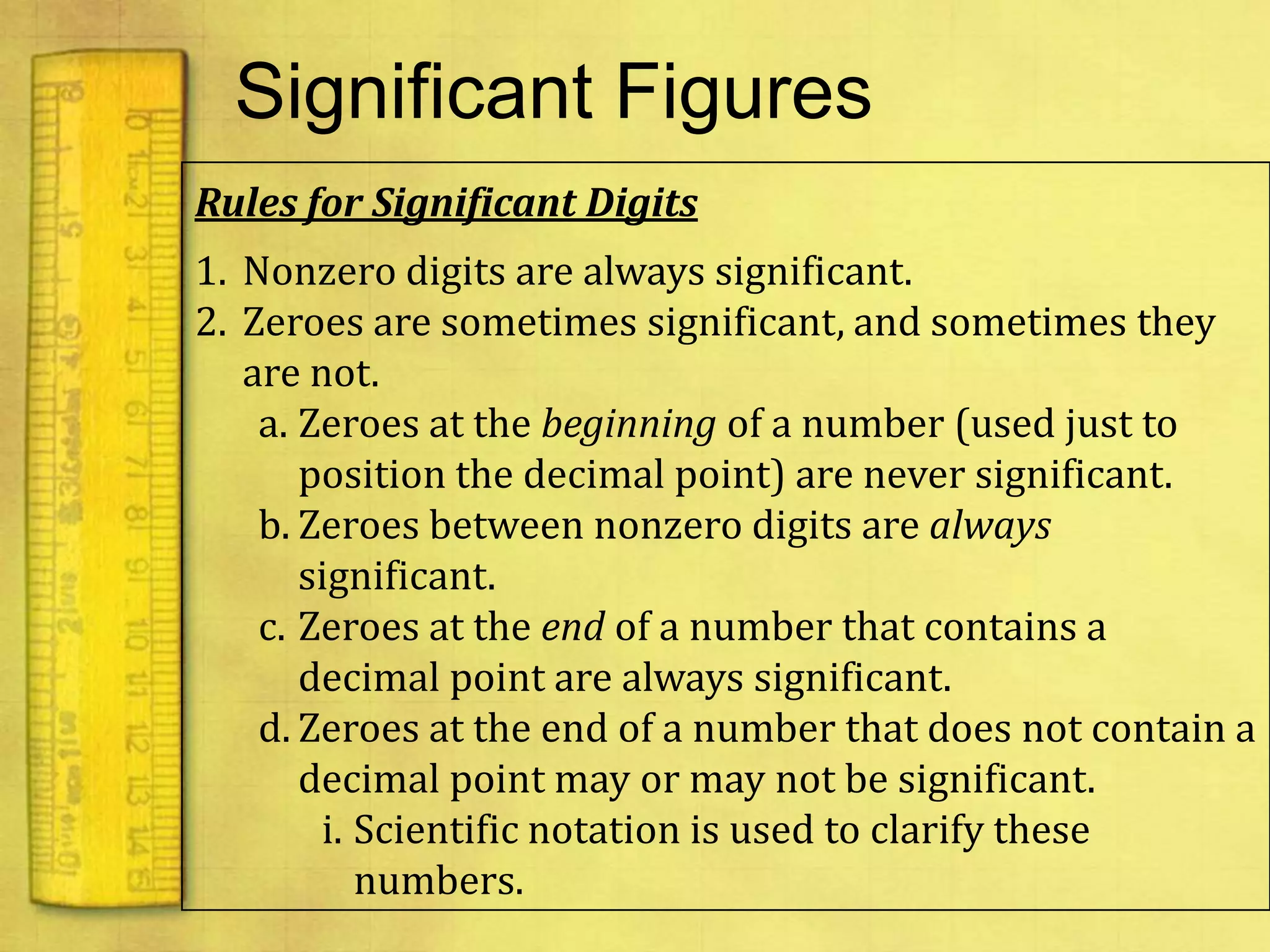
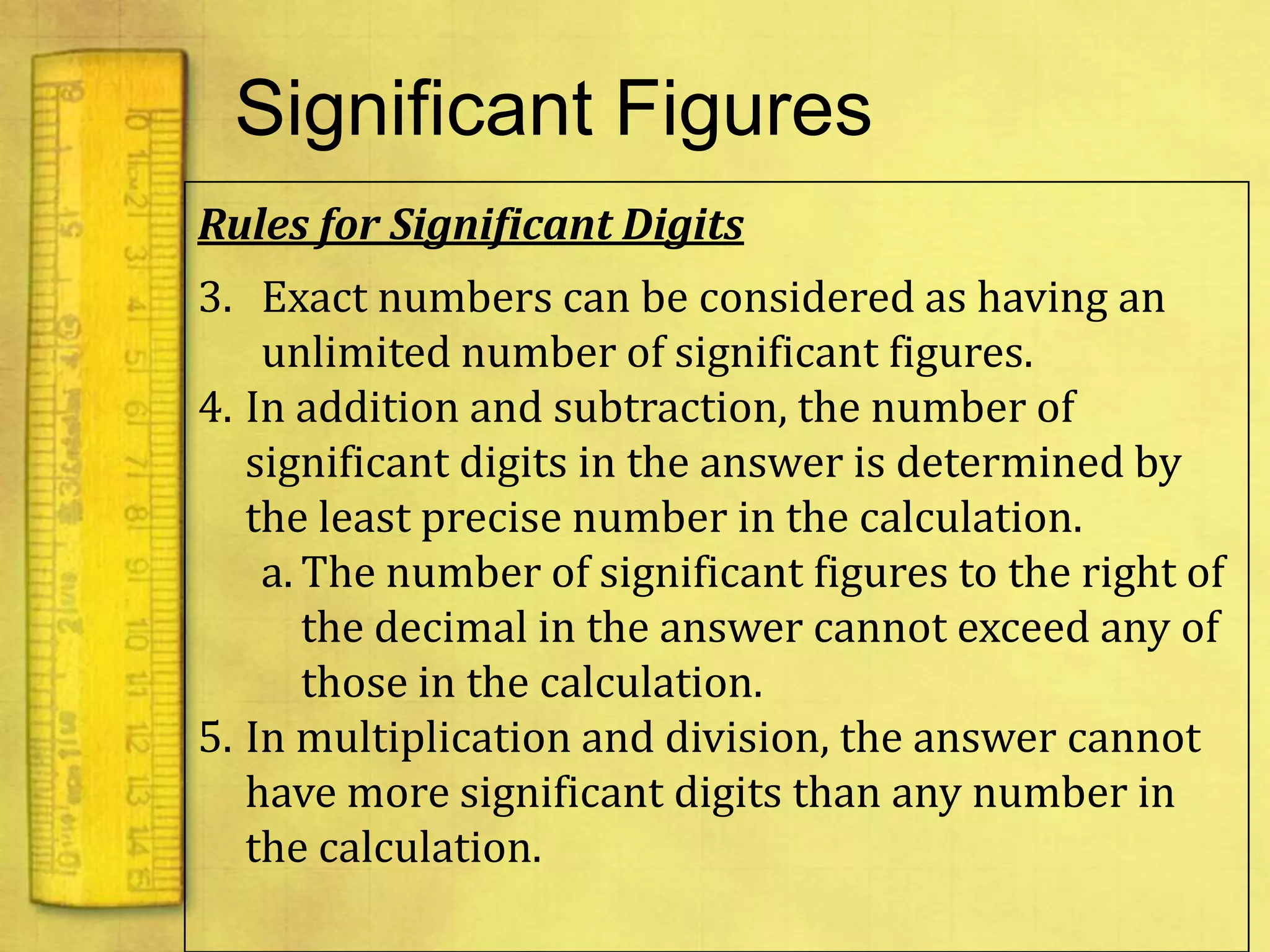
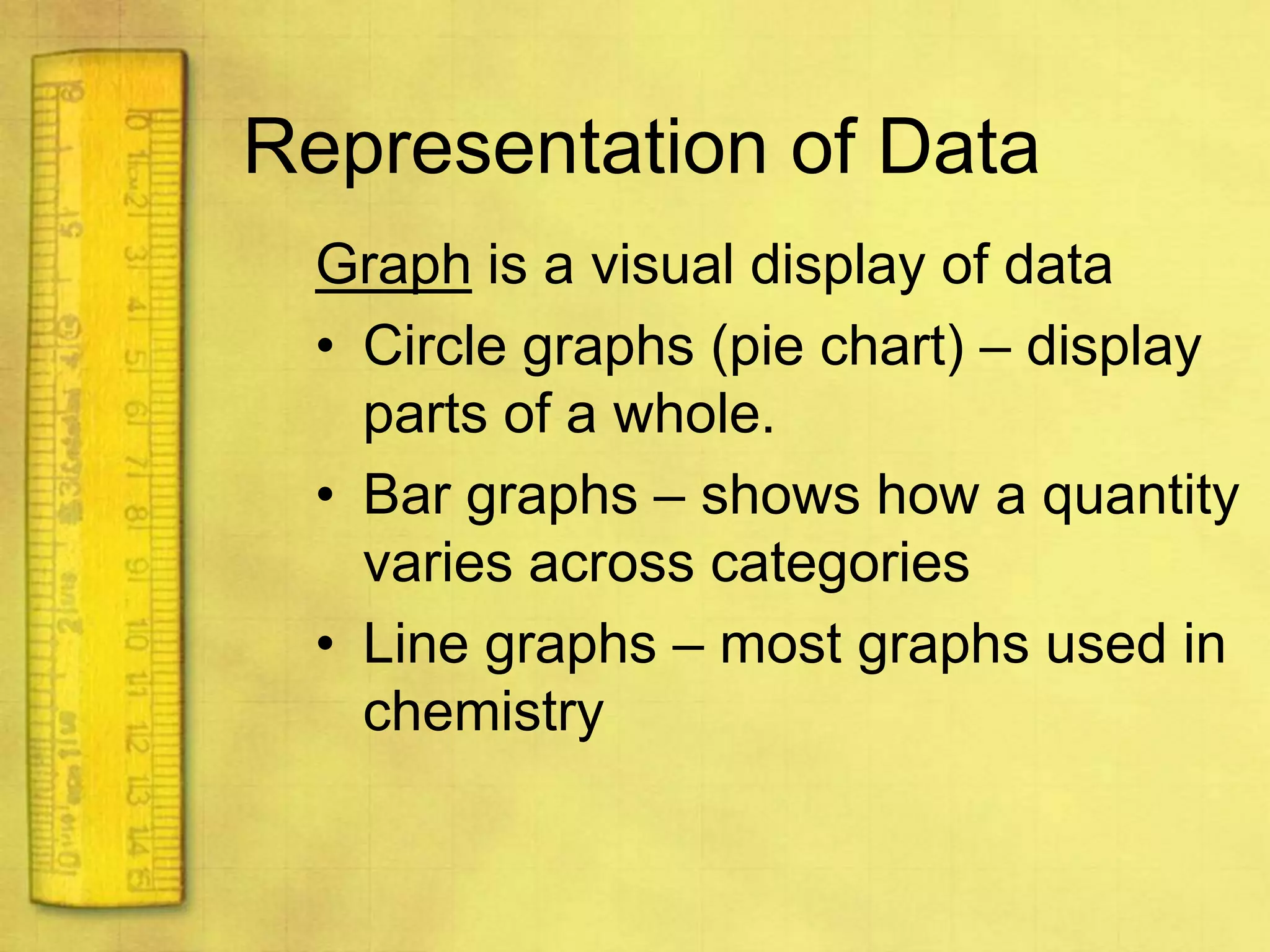
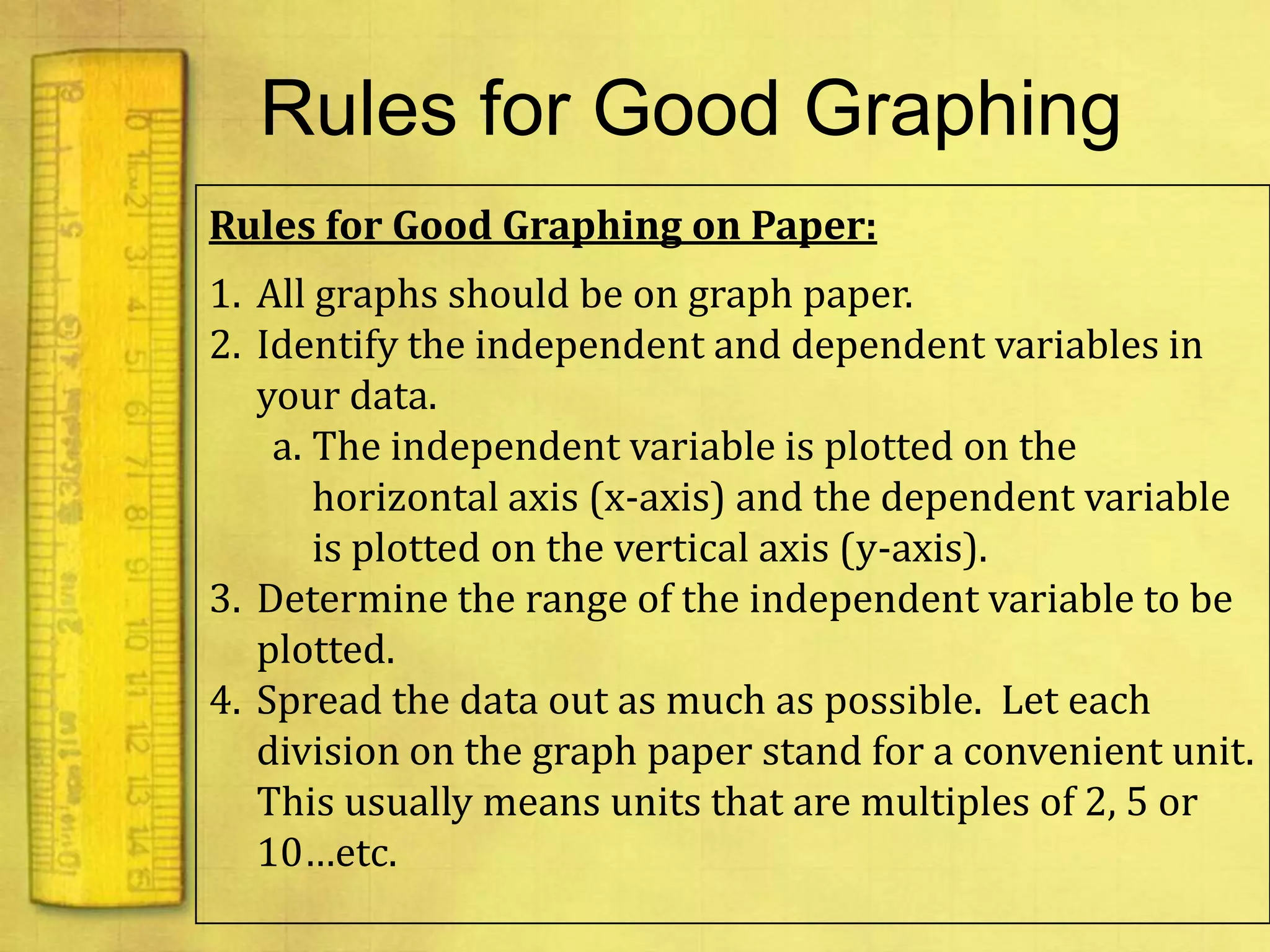
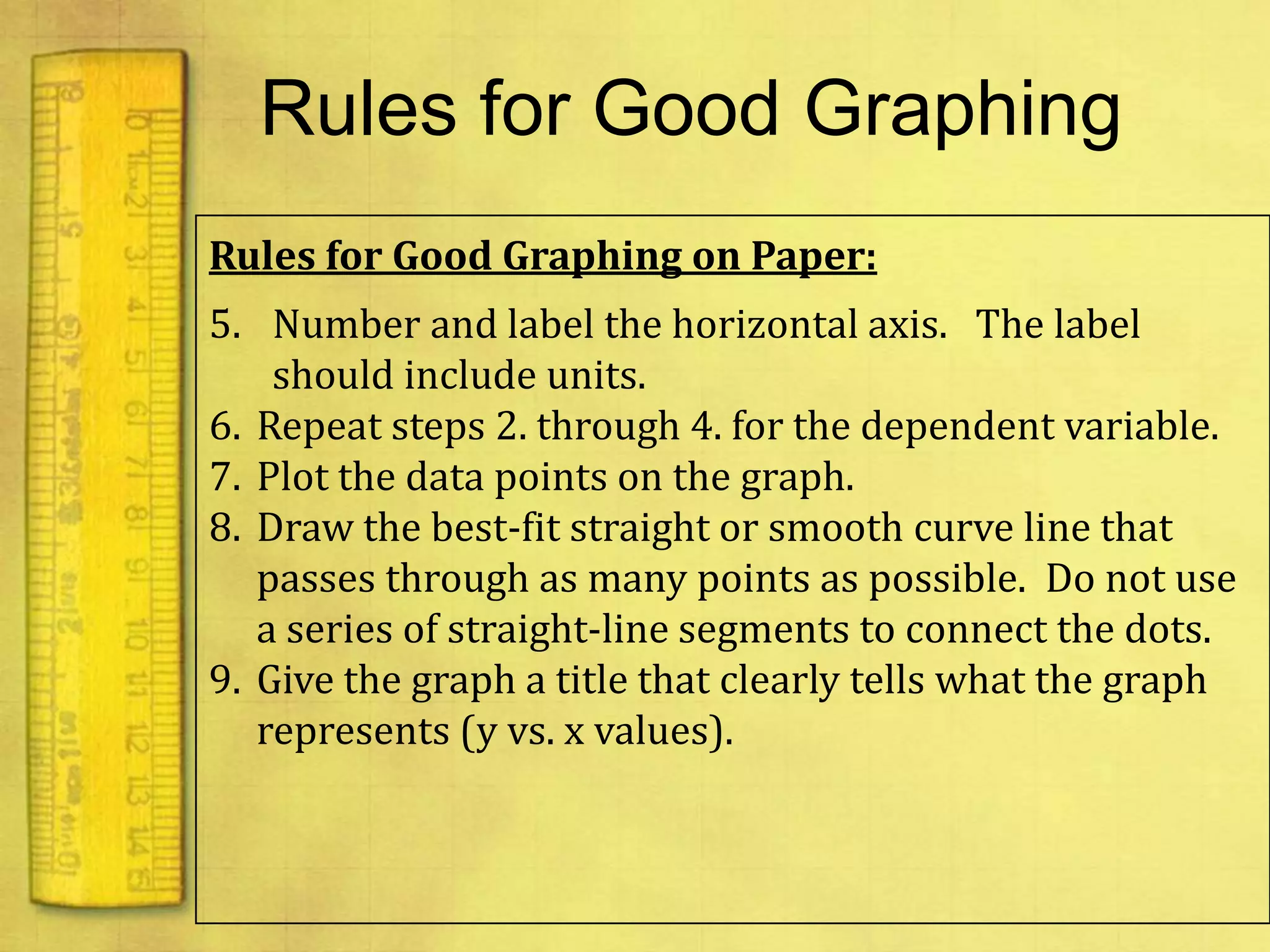
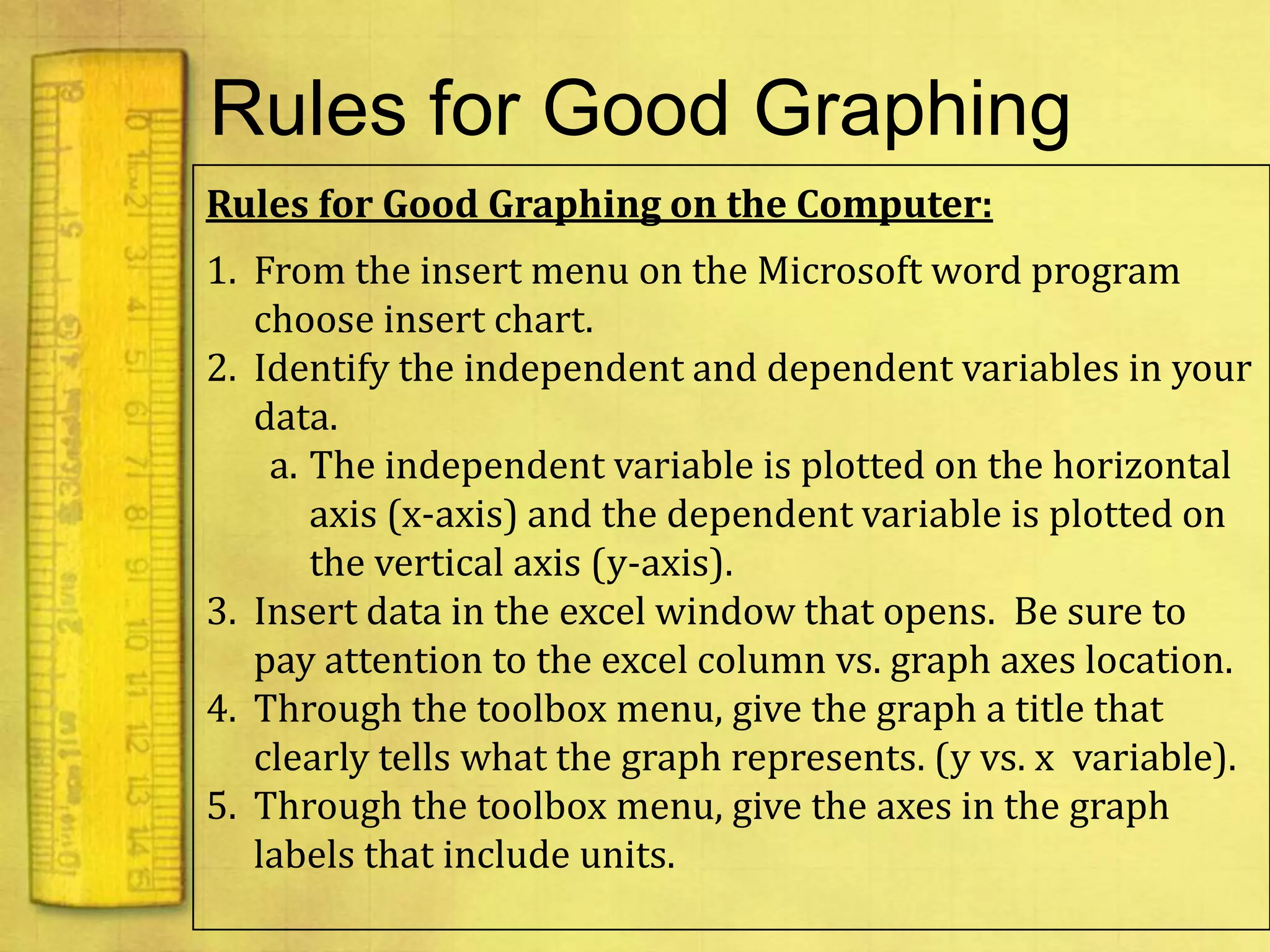
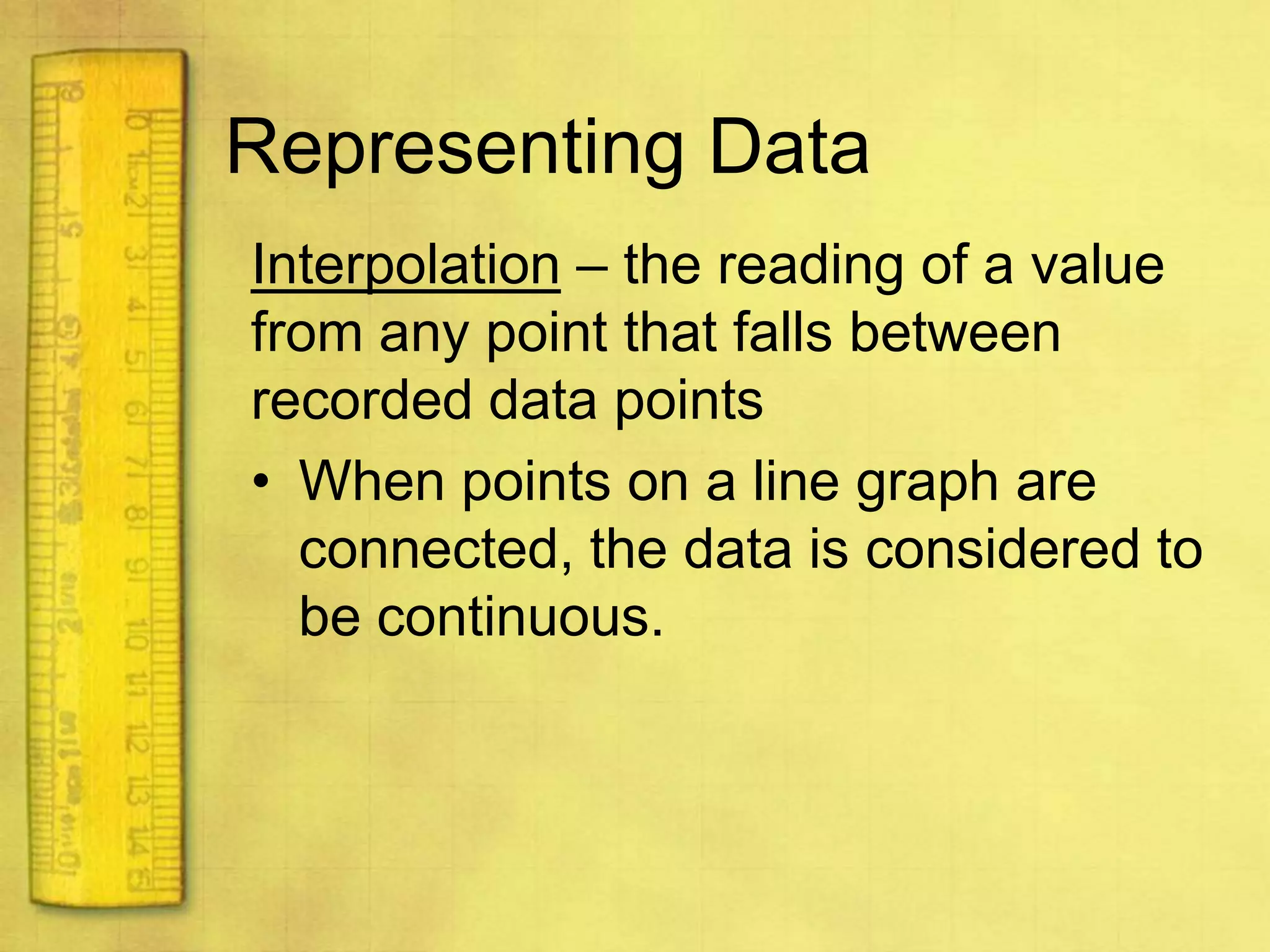
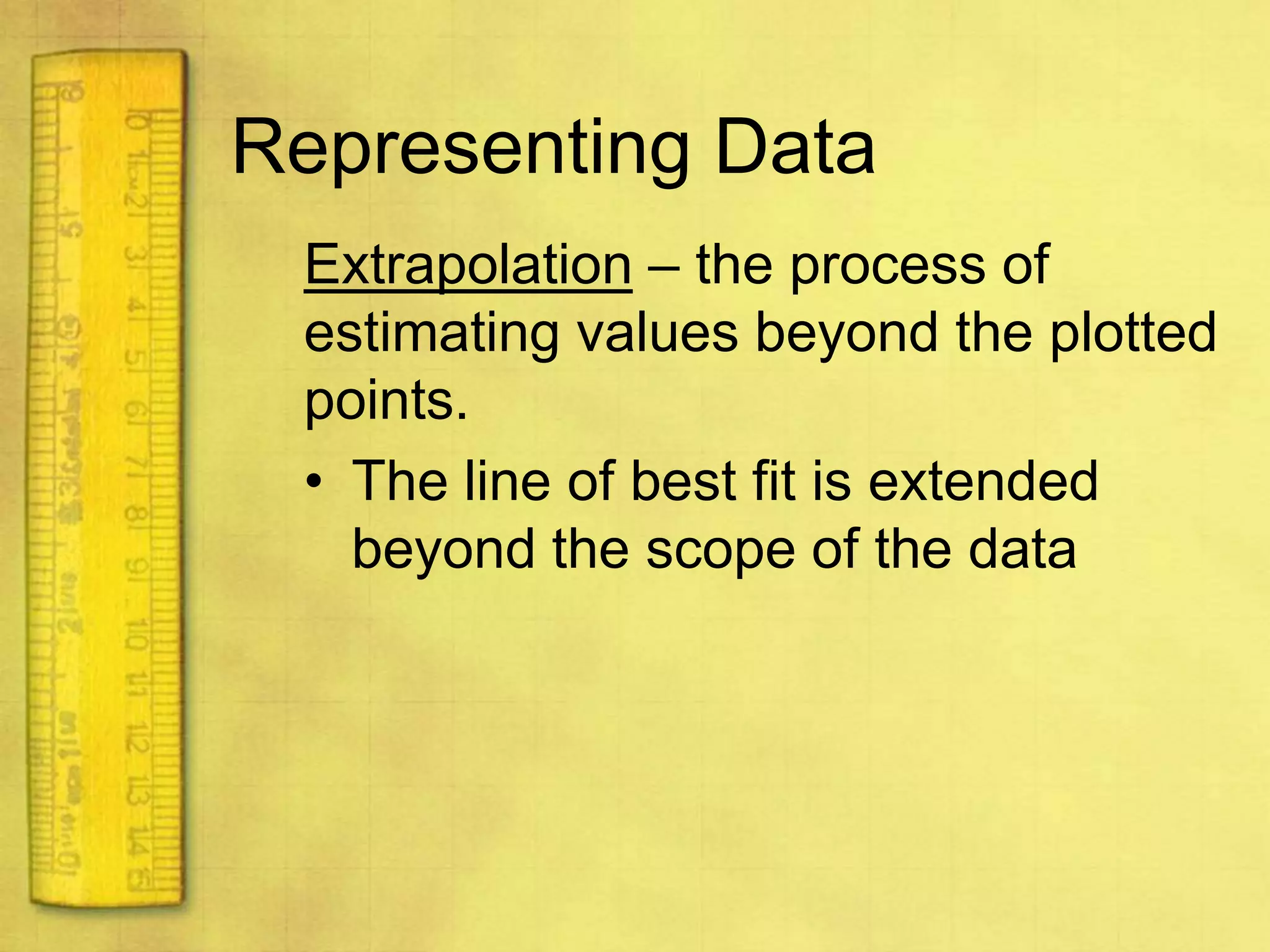
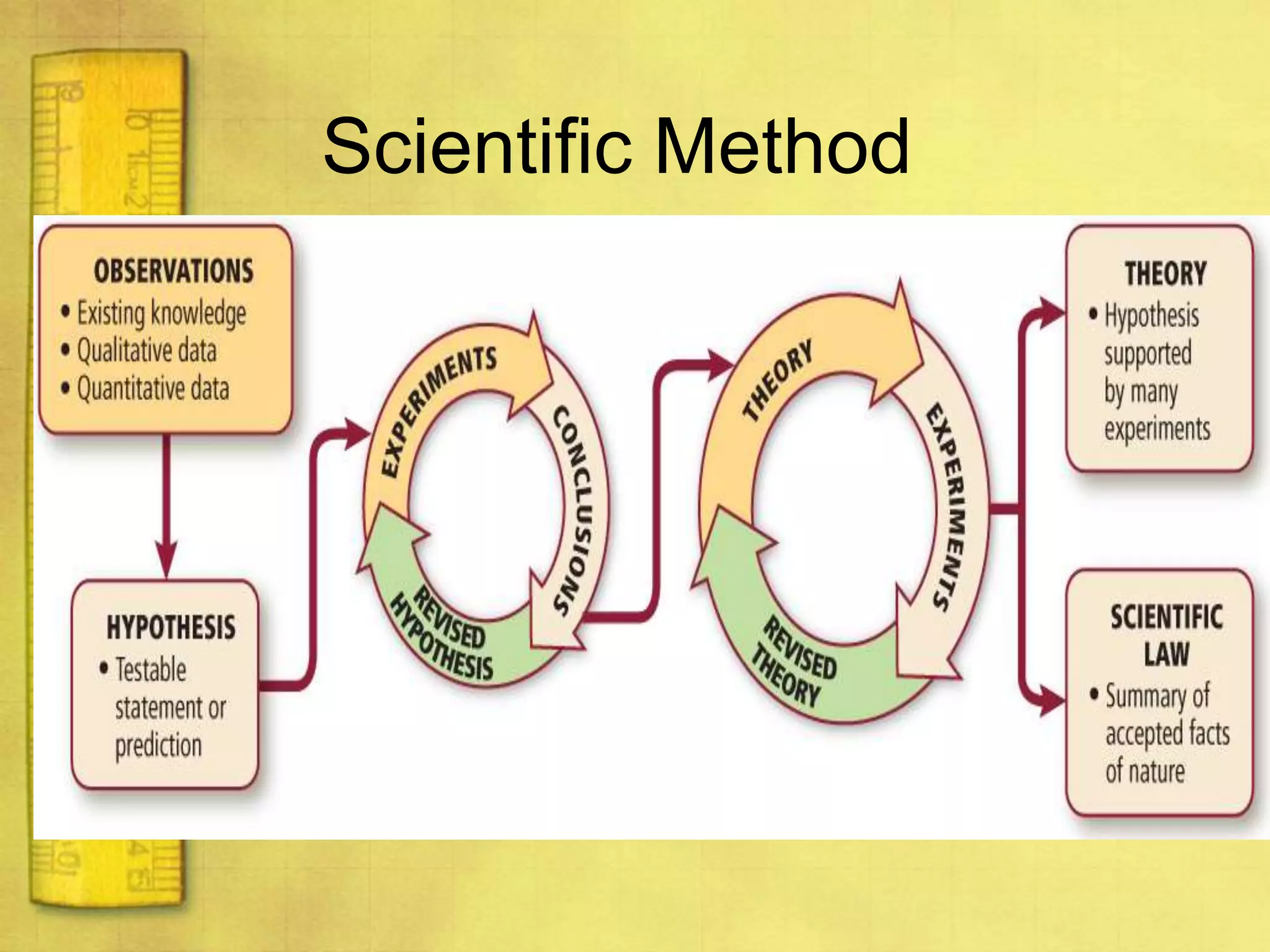
Data can provide information about the natural world. It can be collected through observation and measurement, and expressed quantitatively or qualitatively. Data patterns and relationships can be identified through graphing and analysis. The scientific method involves making observations, developing hypotheses, designing experiments to test hypotheses, and drawing conclusions. Data is key to advancing scientific theories and applying research findings.