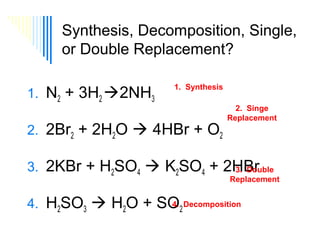
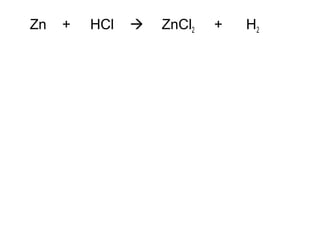
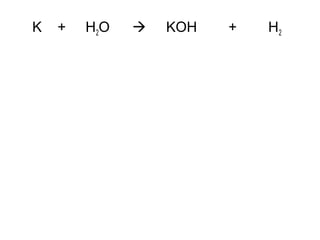
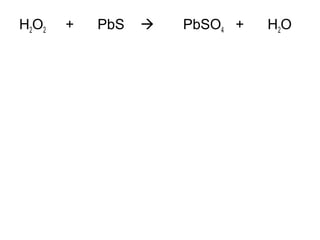
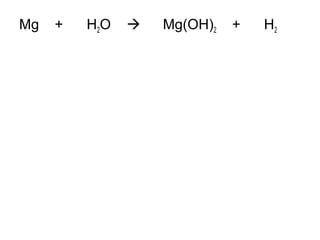
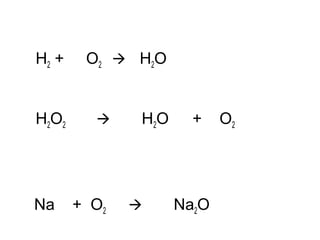
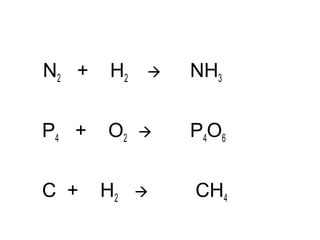
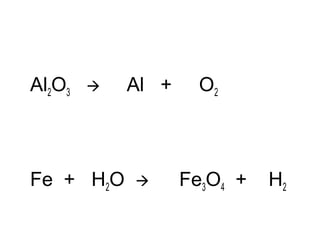
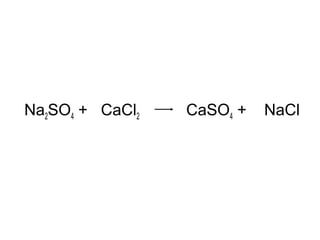
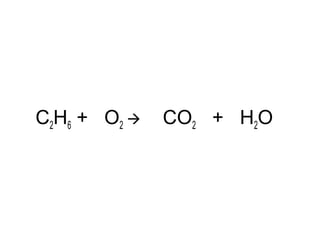The document discusses four types of chemical reactions: synthesis, decomposition, single replacement, and double replacement. It provides examples of each type of reaction and describes how to balance chemical equations by ensuring the same number and type of atoms are on both sides of the equation. The key is only changing the coefficients in front of the formulas and not the subscripts inside the formulas.