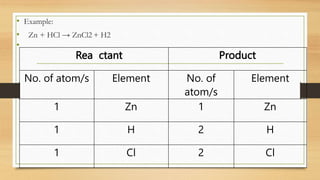
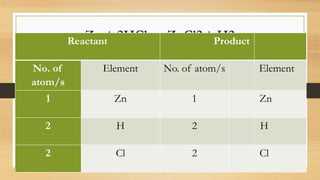
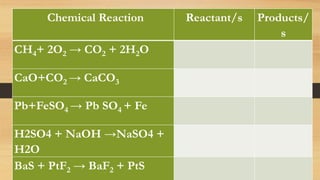
The document discusses chemical reactions, including defining a chemical reaction as the rearrangement of atoms to form new substances. It identifies indicators that a reaction occurred and explains that reactions are represented by balanced chemical equations. The document provides steps for balancing equations, including identifying elements and applying the law of conservation of mass. It also gives examples of balanced and unbalanced equations and an activity to practice balancing equations.