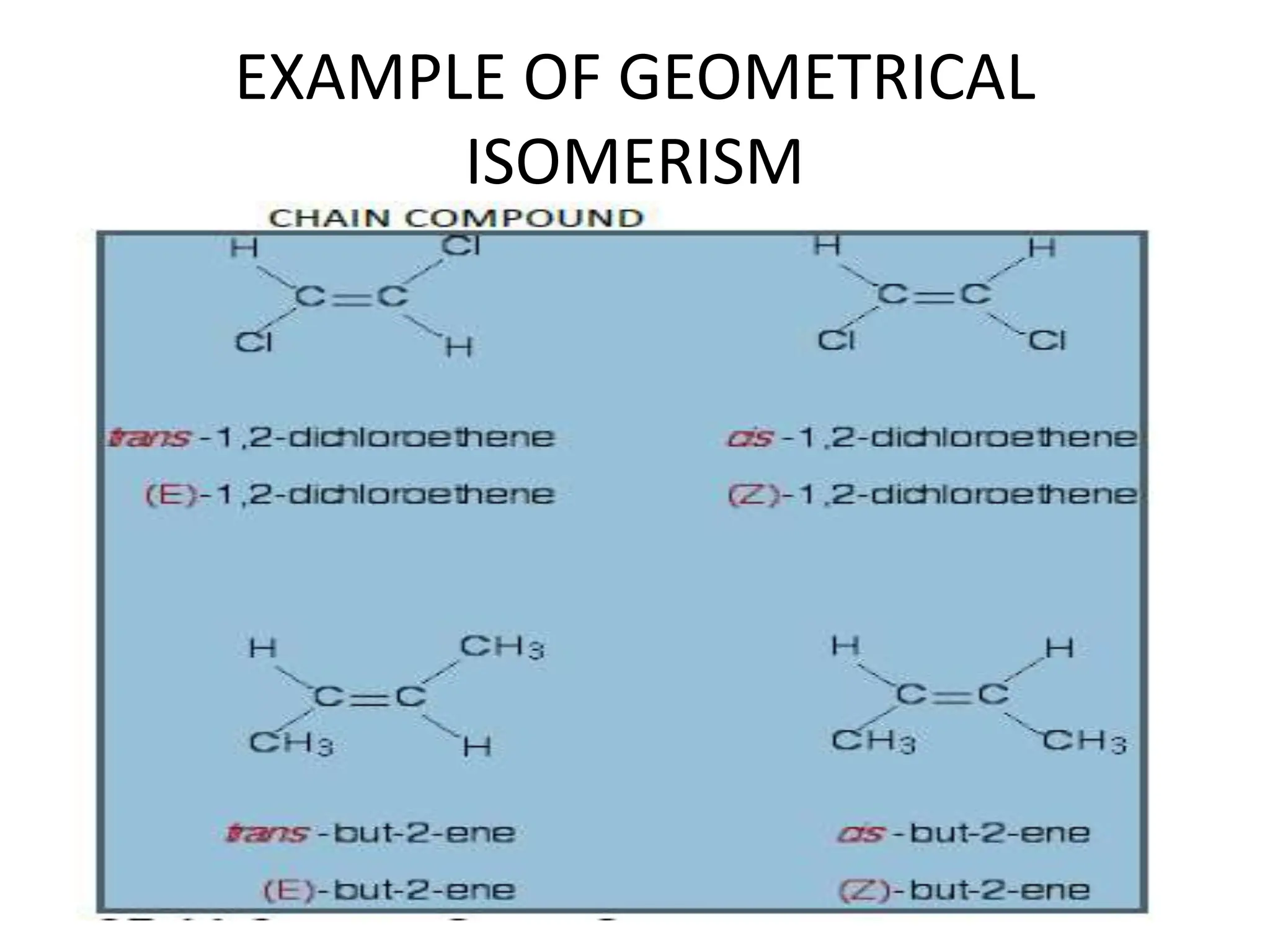
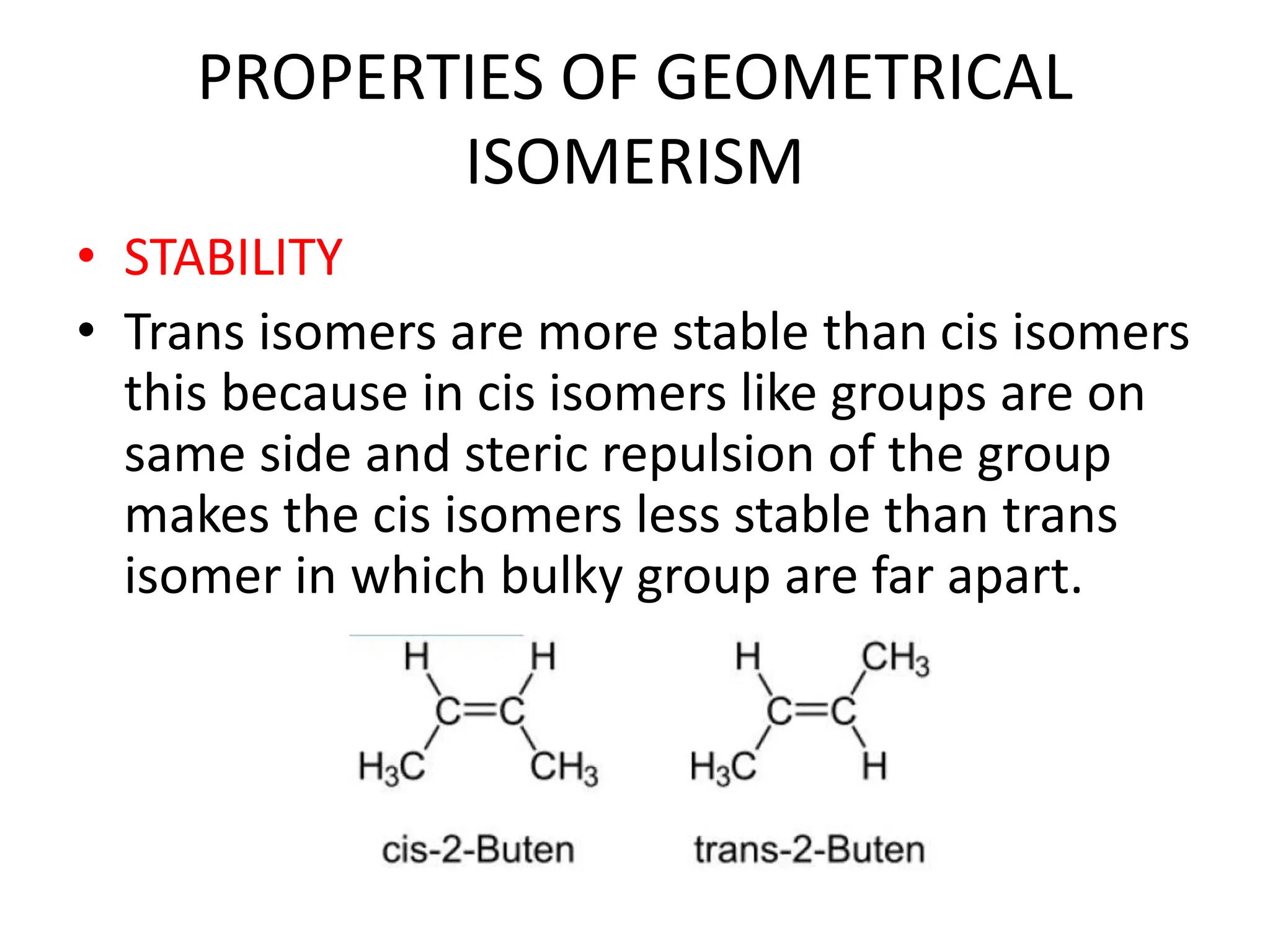
Geometric isomerism results from restrictions in the rotation of double bonds in acyclic compounds and single bonds in cyclic compounds. This restriction leads to cis and trans isomers, where cis has similar groups on the same side of the bond and trans has them on opposite sides. The E/Z system is used to name more complex geometric isomers based on priority of substituents. Trans isomers are typically more stable than cis due to less steric hindrance between bulky groups. Geometric isomers can be interconverted by heating to provide enough energy to break the pi bond or by absorption of light.