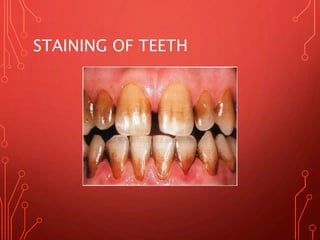
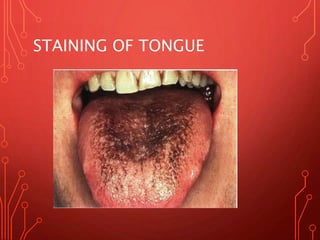
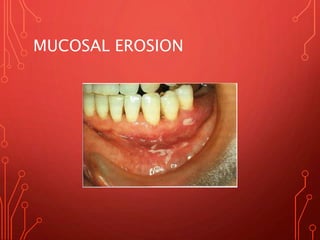
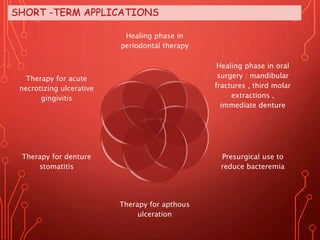
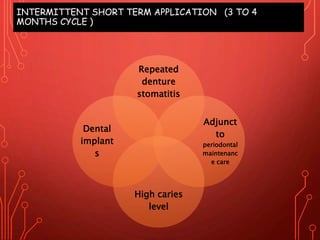
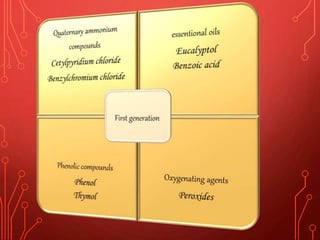
The document discusses various chemical agents and vehicles used for dental plaque control, classifying them into different generations based on their efficacy and properties. It covers specific agents such as antibiotics, bioguanides, and essential oils along with their mechanisms, applications, and associated benefits or side effects. Additionally, it details delivery methods like toothpastes, mouthwashes, and sprays, emphasizing their roles in oral health management.













![TOOTHPASTE
A dentifrice is a substance used with a tooth brush for
purpose of cleaning the accessible surface of teeth and
removing dental plaque, materia alba and food debris
Basically, used for applying specific agents to tooth
surface for preventive or therapeutic purposes
It is derived from dens= [tooth]
Fricare= [to rub] (WEBSTER’S)](https://image.slidesharecdn.com/chemical-plaque-control1-210128040929/85/Chemical-Plaque-Control-15-320.jpg)