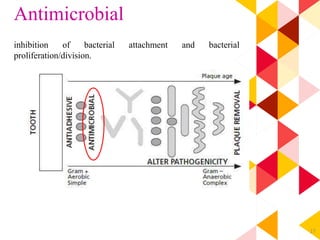
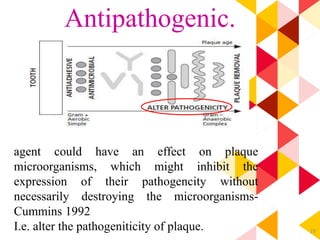
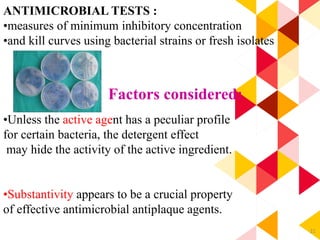
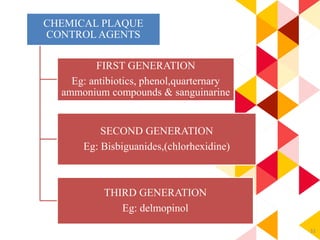
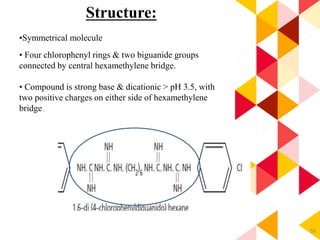
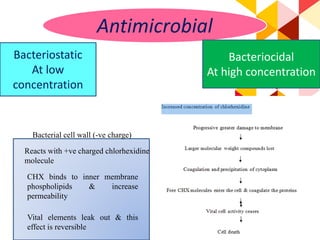
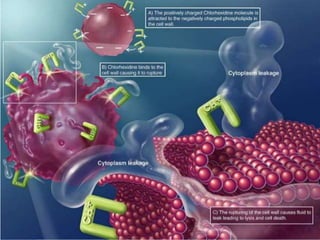
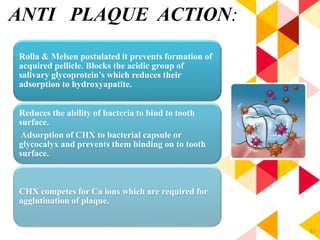
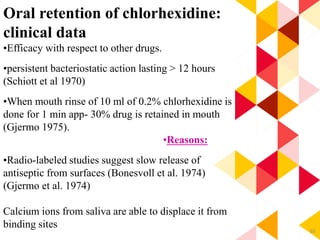
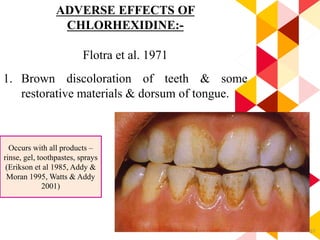
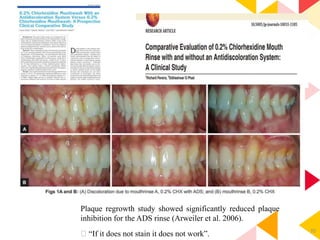
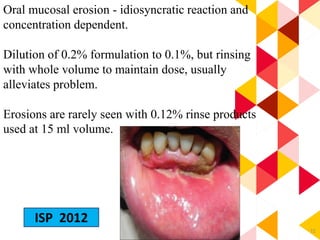
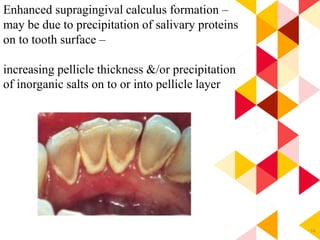
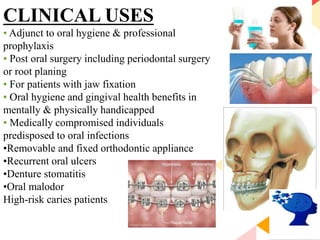
This document discusses chemical plaque control agents. It begins with an introduction and overview of the concept and history of chemical plaque control. It then covers the rationale, approaches, and methods for evaluating antiplaque agents. Ideal properties and groups of agents used are described, including bisbiguanides like chlorhexidine, which are considered the most effective second generation agents. Recent advances and future trends in chemical plaque control agents are also mentioned.