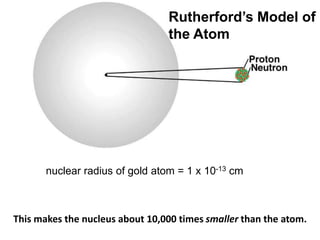
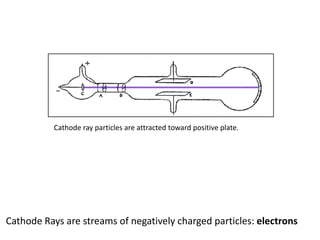
This document summarizes the history of the atomic model from ancient Greek philosophers to modern particle physics. It describes how Democritus first proposed the idea of indivisible atoms in 460 BC. In the early 1900s, J.J. Thomson's plum pudding model depicted atoms as electrons scattered within a uniform positive charge. Rutherford's gold foil experiment in 1910 showed that the positive charge and most of the mass are concentrated in a tiny nucleus. Neutrons were discovered by Chadwick in 1932. Modern atomic structure consists of a small, dense nucleus of protons and neutrons surrounded by electrons.











![Atoms’ positive charge is concentrated in a nucleus 2.2
The Rutherford experiment
“It was about as credible as if you had fired a 15-inch [artillery] shell
at a piece of paper and it came back and hit you.” -- Rutherford](https://image.slidesharecdn.com/chem1-lecture1-2-atomic-structure1-150113091926-conversion-gate01/85/Chem1-lecture1-2-atomic-structure-1-16-320.jpg)