The periodic table developed over time as scientists discovered more elements and recognized patterns in their properties.
- In the 4th century BC, Aristotle proposed that all matter was made of combinations of earth, water, air and fire. Democritus later suggested that matter was made of tiny particles called atoms.
- In the 19th century, scientists like Berzelius, Dobereiner, and de Chancourtois arranged the known elements in order of atomic weight and recognized patterns. Newlands proposed the Law of Octaves in 1864.
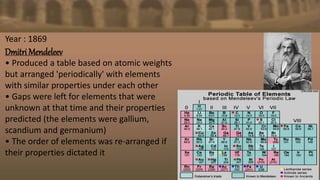
- In 1869, Mendeleev produced the first recognizable periodic table, arranging elements by atomic weight but adjusting order based on properties, and leaving gaps for undiscovered elements. This